Samurai Wasp Trissolcus japonicus (Ashmead) (Insecta: Hymenoptera: Scelionidae: Telenominae)
The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
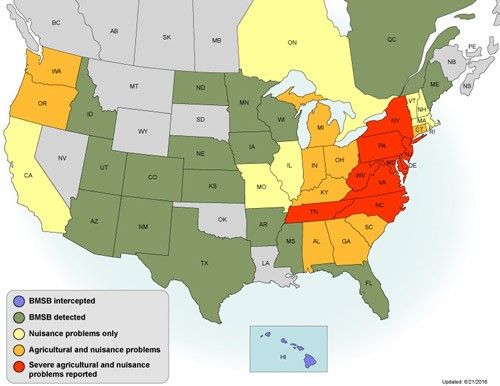
The Samurai wasp, Trissolcus japonicus (Ashmead), is an egg parasitoid of the brown marmorated stink bug, Halyomorpha halys Stål (Hemiptera: Pentatomidae) (McKellar and Engel 2012). The brown marmorated stink bug was first confirmed on Adams Island in Allentown, Pennsylvania in 1996 (Hoebeke and Carter 2003) and has since been detected in 43 states and two Canadian Provinces (Leskey 2016). This stink bug, native to eastern Asia, is a polyphagous pest of ornamental plants, fruit trees, and vegetable crops. The brown marmorated stink bug has become a significant agricultural pest causing economic losses (Rice et al. 2014). The pest usually has one to two generations per year, but may have more in warmer southern areas. A female brown marmorated stink bug may lay as many as 400 eggs in her lifetime. A Trissolcus japonicus female will oviposit her eggs inside the eggs of the brown marmorated stink bug, and the wasp larva develops inside the stink bug egg.
Credit: Tracy Leskey, USDA ARS
Synonymy
According to Talamas et al. (2013), Trissolcus halyomorphae Yang is a junior synonym of Trissolcus japonicus (Ashmead).
Distribution
Trissolcus japonicus is native to China, Japan, and South Korea, where the brown marmorated stink bug is native. Researchers began searching in these areas in 2005 to identify candidate biological control agents for the brown marmorated stink bug (Dieckhoff and Hoelmer 2014). Several species of were identified as egg parasitoids of the brown marmorated stink bug. The most abundant and widespread of the species collected were Trissolcus japonicus and Trissolcus cultratus (Mayr) (Dieckhoff and Hoelmer 2014). Both have been under study in US quarantine facilities since 2007 to evaluate their efficacy as possible classical biological control agents for the brown marmorated stink bug and host specificity with regard to native stink bugs (Suszkiw 2014). Adventive, wild populations of Trissolcus japonicus were discovered in Beltsville, Maryland in 2014 (Talamas et al. 2015b), at several additional nearby sites in Maryland, Washington D.C., and in Winchester, Virginia, during 2015 (Jentsch 2015: unpublished survey data) and in Vancouver, Washington, in August 2015 (Milnes et al. 2016). It is speculated that these wild populations of Trissolcus japonicus may have arrived within stink bug egg masses on plant cargo shipped from Asia.
Description
Adults
Trissolcus japonicus adults are small black wasps (1.0–2.0 mm long) (Dieckhoff and Hoelmer 2014). The size of the wasp depends on the size of the host egg from which it emerged (Medal and Smith 2015). The female is slightly larger than the male by 0.1–0.2 mm. The head is large, broader than the thorax, and glossy with a few punctures. The 11-segmented antennae are brown-black, the scape and pedicel yellow-brown. The mandibles are reddish brown. The legs are mostly black with the tibia and tarsi yellow-brown to pale yellow (Hirashima et al. 1981). The wings are transparent with pale yellow veins. The abdomen is broadly oval and longer than the thorax (Ashmead 1904). The ovipositor is barely protruding. For a description that will separate Trissolcus japonicus from other Trissolcus species, review the diagnosis by Talamas et al. (2015a).

Credit: Elijah J. Talamas, ARS USDA

Credit: Elijah J. Talamas, ARS USDA

Credit: Elijah J. Talamas, ARS USDA

Credit: Elijah J. Talamas, ARS USDA
Eggs
Trissolcus japonicus deposits eggs singly within eggs of the brown marmorated stink bug. An average female Trissolcus japonicus contains 42 eggs in her ovaries at any one time, enabling her to parasitize an entire brown marmorated stink bug egg mass (Yang et al. 2009). Females chemically mark the egg in which they oviposit and will defend the egg clutch against other rival parasitoids. Males will commonly emerge first, wait atop the egg mass for the female to emerge, and then mate with females as they emerge. The female to male ratio is approximately 5.5:1.0 (Yang et al. 2009). The parasitoid can have up to ten generations per year, whereas their host generally has one to two generations a year.

Credit: Elijah J. Talamas, ARS USDA
Click here to view a video of the life cycle of Trissolcus japonicus inside BMSB (video by Chris Hedstrom, published by Entomology Society of America, 2012).
Hosts
Trissolcus japonicus is known to attack the brown marmorated stink bug, Halyomorpha halys, and a native species, Podisus maculiventris (Say), and is under study to determine if it will parasitize other native stink bugs in the US. Non-target species evaluated in China in laboratory choice tests and field surveys concluded that the ecological host range of Trissolcus japonicus contains several other Pentatomidae species, including Plautia fimbriata (Fabr.), Erthesina fullo (Thunberg), and Dolycorisbaccarum (L.) (Haye 2014). The parasitoid has also been recorded from Glaucias subpunctatus (Walker) (Matsuo et al. 2016).
Selected References
Ashmead WH. 1904. Descriptions of new Hymenoptera from Japan 1. Journal of the New York Entomological Society 12: 65-–84.
Bergman EJ, Venugopal PD, Martinson HM, Raupp MJ, Shrewsbury PM. 2016. Host plant use by the invasive Halyomorpha halys (Stål) on woody ornamental trees and shrubs PLOS ONE 11: e0149975.
Dieckhoff C, Hoelmer KA. 2014. Classic biological control: Status of host range tests with Asian egg parasitoids. Northeastern IPM Center.
Haye T. 2014. Seasonal field parasitism of Halyomorpha halys and co-occurring non-target species in China. Brown Marmorated Stink Bug Working Group Meeting, Georgetown, DE.
Hirashima Y, Yamagishi K, Cora J, Johnson N. 1981. Redescriptions of the types of some Japanese Scelionidae preserved in the United States National Museum (Hymenoptera: Proctotrupoidea). Journal of the Faculty of Agriculture, Kyushu University 25: 153–159.
Hoebeke ER, Carter ME. 2003. Halyomorpha halys Stål (Hemiptera: Pentatomidae) a polyphagous plant pest from Asia newly detected in North America. Proceedings of the Entomological Society of Washington 105: 225–237.
Jentsch PJ. 2015. Third find of T. japonicus: Winchester, Virginia, June 2015. The Jentsch Lab blog.
Leskey T. 2016. Stop BMSB Biology, ecology, and management of brown marmorated stinkbug in specialty crops. http://stopbmsb.org (14 November 2016).
Matsuo K, Honda T, Itoyama K, Toyama M, and Hirose Y. 2016. Discovery of three egg parasitoid species attacking the shield bug Glaucias subpunctatus (Hemiptera: Pentatomidae). Japanese Journal of Applied Entomology and Zoology (in Japanese, English abstr.) 60: 43–45.
McKellar RC, Engel MS. (2012). Hymenoptera in Canadian Cretaceous amber (Insecta). Cretaceous Research 35: 258–279. doi: 10.1016/j.cretres.2011.12.009.
Medal J, Smith, T. 2015. Reversible polyphenism in Trissolcus japonicus (Hymenoptera: Platygastridae) induced by stink bug egg size. Journal of Entomological Science 50:363–366.
Milnes JM, Wiman NG, Talamas EJ, Brunner JF, Hoelmer KA, Buffington ML, Beers EH. 2016. Discovery of an exotic egg parasitoid of the brown marmorated stink bug, Halyomorpha halys (Stål) in the Pacific Northwest. Proceedings of the Entomological Society of Washington 118: 466–470.
Rice K, Bergh C, Bergman E, Biddinger D, Dieckhoff C, Dively G, Fraser H, Gariepy T, Hamilton G, Haye T, Herbert A, Hoelmer K, Hooks C, Jones A, Krawczyk G, Kuhar T, Leskey T, Mitchell W, Neilson AL, Pfeiffer D, Raupp M, Rodriguez-Saona C, Shearer P, Shrewsbury P, Tooker J, Venugopal D, Whalen J, Wiman N. 2014. Biology, ecology, and management of brown marmorated stink bug (Halyomorpha halys). Journal of Integrated Pest Management 5: A1–A13.
Suszkiw J. 2014. Up close and personal with tiny, beneficial wasps. Agricultural Research 62: 18–19.
Talamas EJ, Buffington M, Hoelmer K. 2013. New synonymy of Trissolcus halyomorphae Yang. Journal of Hymenoptera Research 33: 113–117. doi: 10.3897/JHR.33.5627
Talamas EJ, Johnson NF, Buffington, ML. 2015a. Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae). Journal of Hymenoptera Research 43: 45–110.
Talamas EJ, Herlihy MV, Dieckhoff C, Hoelmer KA, Buffington M, Bon MC, Weber DC. 2015b. Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. Journal of Hymenoptera Research 43: 119–128.
Yang Z-Q, Yao Y-X, Qiu L-F, Li Z-X. 2009. A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with comments on its biology. Annuals of the Entomological Society of America 102: 39–47.


