Citrus Nematode, Tylenchulus semipenetrans (Cobb 1913) (Nematoda: Secernentea: Tylenchida: Tylenchulidae: Tylenchulinae)
Introduction
The citrus nematode was first discovered infecting citrus in California (Thomas 1913). Later that year, Nathan Cobb (1913) described this nematode as a new species, Tylenchulus semipenetrans, which then was identified as the causal agent of slow decline in citrus. Since its discovery, T. semipenetrans has been found in every citrus growing region of the world (Duncan 2005). In the United States, field infestations involve 50%–90% of the citrus orchards in Arizona, California, Florida, and Texas, and localized vineyards in California (Van Gundy and Meagher 1977; Heald and O'Bannon 1987; Duncan 2005).
Distribution
Tylenchulus semipenetrans evolved in the Far East with citrus and was disseminated to many citrus growing areas of the world with nematode-infected, propagative plant material. Higher populations are commonly found in citrus orchards established in finely-textured soils or in sandy soils with high organic matter content. Fluctuations in soil salinity from high to low favors nematode reproduction, while sandy soils poor in organic matter hinder population increase (Timmer et al. 2003).
Description
Superficially, T. semipenetrans J2 appear similar to those of Meloidogyne (root-knot nematodes) because of their narrow and tapered posterior body portion (Figure 1).
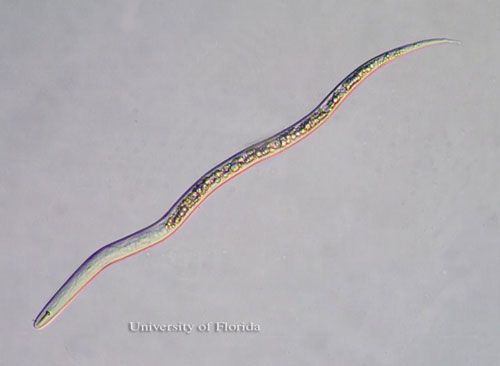
Credit: Nicholas S. Sekora, University of Florida
Further scrutiny reveals readily apparent distinctions between T. semipenetrans and Meloidogyne J2s. Tylenchulus semipenetrans male and female J2s differ from those of Meloidogyne by the presence of a stronger stylet, more posterior excretory pore, and absence of pharyngeal overlap. A hyaline band corresponding to the copulatory system in the posterior portion of the body characterizes T. semipenetrans J2 males. This band is not present in T. semipenetrans J2 females or Meloidogyne J2 of either sex.
Vermiform and mobile T. semipenetrans mature males are found in the soil or in the egg masses. Mature females are found attached to roots and are typically covered by soil particles and debris that stick to the gelatinous matrix, helping to protect the exposed portion of their body and eggs. The female posterior portion of the body protruding from the root surface is swollen and enlarged and ends in a finger-like projection, while the elongate and not swollen anterior portion of the body remains hidden and embedded in the cortical parenchyma.
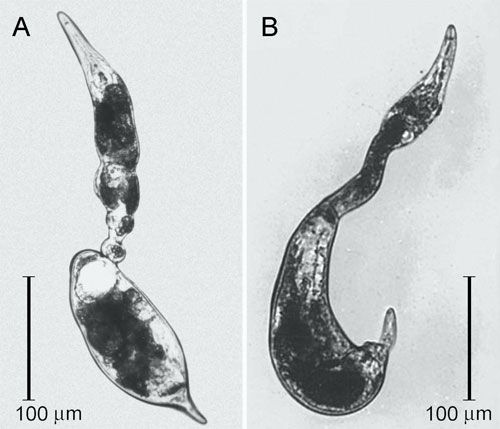
Credit: Courtesy of Inserra et al. (1988).
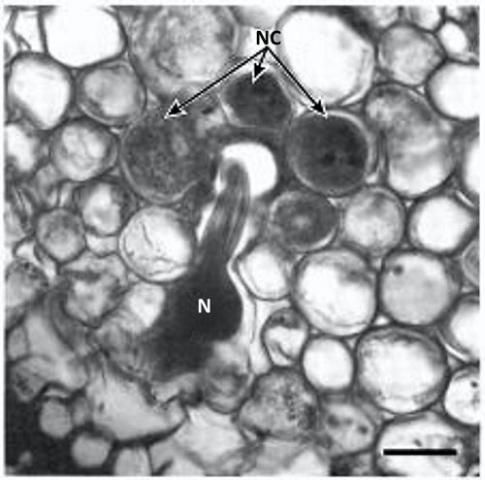
Credit: Courtesy of N. Vovlas
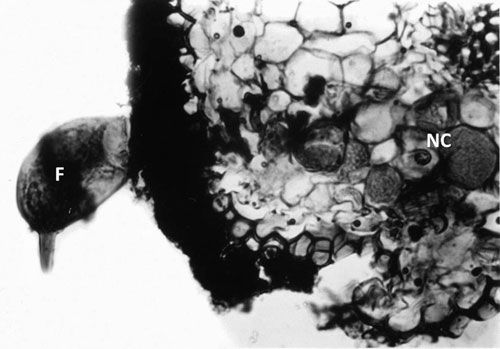
Credit: Courtesy of O'Bannon and Esser (1985)
Life Cycle and Biology
Tylenchulus semipenetrans is a dimorphic species that exhibits sexual dimorphism (male and female individuals) at both the juvenile and adult stage. The life cycle of T. semipenetrans is typical of plant-parasitic nematodes beginning as an egg, which contains the first stage juvenile (J1). This stage molts in the egg into the second-stage juvenile (J2) that hatches and searches for host roots. The motile and vermiform J2 female molts into the vermiform J3 and J4, and finally into the sedentary adult. The development of female juveniles into adults requires feeding on the epidermis and superficial layers of the cortical parenchyma of the roots. The immature female begins to penetrate the outer surface of the root into the deep cortical layers, usually without reaching the central cylinder (or exceptionally the endodermis). It becomes sedentary and establishes a permanent feeding site consisting of specialized cells (nurse cells), which are the main source of nutrients. With maturation, the posterior portion of its body swells and protrudes from the root surface while its elongate neck and head remains embedded into the cortex. Mature females produce eggs that are embedded in a gelatinous matrix secreted through the excretory pore. The length of the female life cycle from egg to egg ranges from four to eight weeks (Van Gundy 1958).
The development of the J2 male into adult is completed in seven days and does not require feeding. Consequently, the feeding apparatus (stylet and esophagus) of adult males is poorly developed and may be difficult to observe. Tylenchulus semipenetrans is a sexually reproducing species that can occasionally reproduce by facultative parthenogenesis without the need of males.
Hosts
Unlike many nematodes, T. semipenetrans has a restricted host range, which includes citrus, trifoliate orange, grapevines, persimmon, lilac, and olive (Thorne 1961; Baines et al. 1969; Inserra et al. 1994). To date, there have been no reports of T. semipenetrans infecting herbaceous plants (Inserra et al. 1994). Since T. semipenetrans was described, several biotypes with different host preference have been observed and separated according to their ability to parasitize citrus, trifoliate orange, and olive. Currently, three biotypes are accepted, citrus, poncirus, and mediterranean (Inserra et al. 1980; Gottlieb et al. 1986; Verdejo-Lucas 1992; Inserra et al. 1994).
Symptoms
Symptoms of slow decline can vary depending on the level of T. semipenetrans infestation, age of trees and time of infection. Recently planted citrus orchards will not demonstrate symptoms until T. semipenetrans populations increase to high levels (at or above 2000 individuals per 100 cc of soil). Symptoms are more prominent in established orchards as trees are stressed either by suboptimal growing conditions, drought, or root stunting and decay induced by T. semipenetrans infection. Typically, reduced leaf and fruit size, canopy thinning, and exposure of bare crown limbs are the most conspicuous symptoms of slow decline and result in yield suppression (Duncan 2005).

Credit: Nicholas S. Sekora, University of Florida
Economic Importance
Florida is the second largest producer of oranges in the world and leads in grapefruit production. Citrus generates more than $9 billion in revenue annually for the state and covers 620,000 acres (Florida Citrus Mutual). Since invasion and reproduction by the citrus nematode is slower in very sandy, coarse textured soils than in fine textured soils with moderate amounts of clay and silt, the citrus nematode in Florida reaches damaging levels more rapidly in the flat wood soils than in the deep sands of the Florida Ridge.
Root damage is more severe in the arid and high salinity conditions of Arizona, California, and Mediterranean citrus areas than in Florida. The crop losses induced by the nematode in heavily infested Florida groves range 10%–20%, whereas in the arid and high salinity conditions of the western states they can reach 50% (Baines et al. 1962; Duncan 2005). Incorporation of management practices that reduce the damage of T. semipenetrans to citrus crops can greatly improve the production of citrus growers around the world.
Management
Past management practices for T. semipenetrans included soil conditioning and nematicide treatments in both replanting situations and established orchards. Nematicide treatments were widely used in established orchards in the past. However, the use of chemical treatments has been greatly restricted and leaves growers with limited options for managing T. semipenetrans. Currently, the use of resistant (Swingle citrumelo) rootstocks and certified propagative citrus plants free from nematode parasites of citrus are promising strategies for preventing the damage induced by T. semipenetrans to citrus (Kaplan 1981; Ling et al. 2000; Galeano et al. 2003). This practice has greatly reduced the spread of this parasite in new Florida lands free from existing nematode infestations (Lee et al. 1999). Resistant (Ramsey) or moderately resistant (vinfera Dog Ridge) grape rootstocks were also used successfully in California vineyards (Edwards 1989, McKenry personal communication). Planting nematode-certified citrus and grape rootstocks is an excellent practice of sustainable agriculture, which should be adopted also for other fruit crops susceptible to nematode infections.
Selected References
Baines RC, Martin JP, DeWolf TA, Boswell SB, Garber MJ. 1962. Effects of high doses of D-D on soil organisms and the growth and yield of lemon trees. Phytopathology 52: 723.
Baines RC, Miyakawa T, Cameron JW, Small RH. 1969. Infectivity of two biotypes of the citrus nematode on citrus and on some other hosts. Journal of Nematology 1: 150–159.
Duncan LW. 2005. Nematode parasites of citrus. pp. 437–466. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture (Luc M, Sikora RA, Bridge J, eds). CAB International, Wallingford, UK.
Duncan LW. 2009. Managing nematodes in citrus orchards. pp. 135–173. In Integrated Management of Fruit Crops and Forest Nematodes, Springer Science and Business Media, New York, NY.
Edwards M. 1989. Resistance and tolerance of grapevine rootstocks to plant-parasitic nematodes in vineyards in North-East Victoria. Australian Journal of Experimental Agriculture 29: 129–131.
Galeano M, Verdejo-Lucans S, Sorribas FJ, Ornat C, Forner JB, Alcaide A. 2003. New citrus selections from Cleopatra mandarin x Poncirus trifoliata with resistance to Tylenchulus semipenetrans Cobb. Nematology 5: 227–234.
Inserra RN, Vovias N, O'Bannon JH. 1980. A classification of Tylenchulus semipenetrans biotypes. Journal of Nematology 12: 283–287.
Inserra RN, Vovlas N, O'Bannon JH, Esser RP. 1988. Tylenchulus graminis n. sp. and T. palustris n. sp. (Tylenchulidae) from native flora of Florida, with notes on T. semipenetrans and T. furcus. Journal of Nematology 20: 266–287.
Inserra RN, Duncan LW, O'Bannon JH, Fuller SA. 1994. Citrus nematode biotypes and resistant citrus rootstocks in Florida. Nematology Circular No. 205. Florida Department of Agriculture and Consumer Services. Division of Plant Industry.
Kaplan DT. 1981. Characterization of citrus rootstock responses to Tylenchulus semipenetrans (Cobb). Journal of Nematology 13: 492–498.
Lee RF, Lehman PS, Navarro L. 1999. Nursery practices and certification programs for budwood and rootstocks. pp. 35-46. In Citrus Health Management. APS Press, St. Paul, MN.
Ling P, Duncan LW, Deng Z, Dunn D, Hu X, Huang S, Gmitter FG, Jr. 2000. Inheritance of citrus nematode resistance and its linkage with molecular markers. Theoretical Applied Genetics 100: 1010–1017.
O'Bannon JH, Esser RP. 1985. Citrus declines caused by nematodes in Florida. II. Physiological races. Nematology Circular No. 116. Florida Department of Agriculture and Consumer Services, Gainesville, Florida, USA.
Thorne G. 1961. Principles of Nematology. McGraw-Hill Book Company, Inc. New York, NY.
Timmer LW, Garnsey SM, Broadbent P. 2003. Diseases of citrus. pp. 163–196. In Diseases of Tropical Fruit Crops. CAB International, Wallingford, UK.




