Management of Plant-Parasitic Nematodes in Florida Soybean Production1
Nematodes in Soybean Production
Nematodes are non-segmented roundworms that are generally microscopic. They live in animal hosts, soil/plant roots, or water. Nematodes in agricultural systems usually live in the soil and can be divided into three categories: (1) entomopathogenic nematodes that feed on insects; (2) free-living nematodes that feed on bacteria, fungi, or other nematodes and may be beneficial for crop production; and (3) plant-parasitic nematodes that feed only on plants and may drastically suppress growth. Plant-parasitic nematodes can cause serious yield loss to crops including soybeans.
Plant-parasitic nematodes of soybeans reduce yield by decreasing root size and efficiency, leading to shorter shoots. Infected plants are generally stunted with varying degrees of yellowing (chlorosis). They produce fewer pods or soybeans, and, in severe cases, they die. The amount of damage nematodes cause is related to their population densities. The higher the density, the greater the damage.
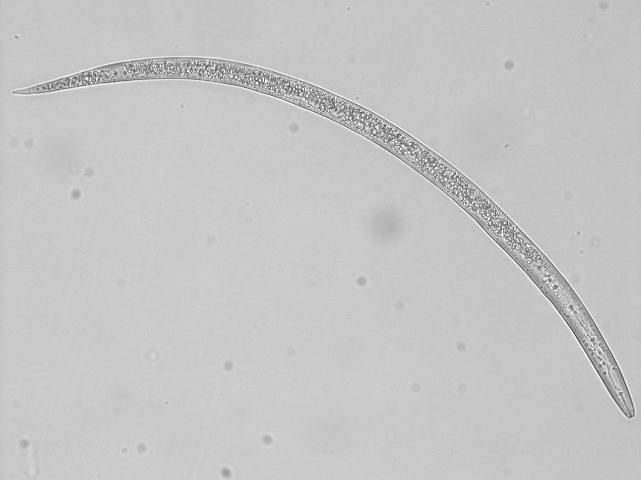
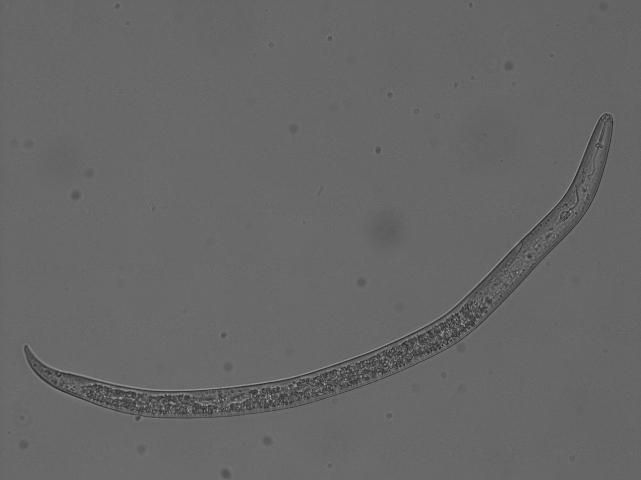
Soybeans are susceptible to most plant-parasitic nematodes in Florida including root-knot (Meloidogyne spp., Figures 1 and 2), reniform (Rotylenchulus reniformis, Figures 3 and 4), sting (Belonolaimus spp.), and lesion (Pratylenchus spp.) nematodes. Most root-knot nematode species in Florida damage soybeans including the three major ones: southern (Meloidogyne incognita), peanut (Meloidogyne arenaria), and Javanese (Meloidogyne javanica) root-knot nematodes. Root-knot nematode is both widespread and highly damaging to soybeans in Florida. Reniform nematode can be damaging, particularly at high densities, but favors heavier soils (80% or less sand), such as those found in the Florida Panhandle. Sting nematode is highly damaging, but is only found in very sandy soils (80% or greater sand, 10% or less clay) with minimal organic matter. Lesion nematode has only moderate damage potential.

Credit: Charles Overstreet, Louisiana State University. Used with permission.
Credit: Zane Grabau, UF/IFAS

Credit: Charles Overstreet, Louisiana State University. Used with permission.
Credit: Zane Grabau, UF/IFAS
Soybean cyst nematode (Heterodera glycines) is a limiting factor for soybean production in much of the United States (Figure 5). It was a serious problem in Florida a number of years ago, but reports of this nematode diminished when soybean acreage diminished because this nematode only damages soybeans and related crops. Soybean cyst nematode can survive many years in the soil, so while its distribution and population levels may have diminished, it is probably still present in Florida.
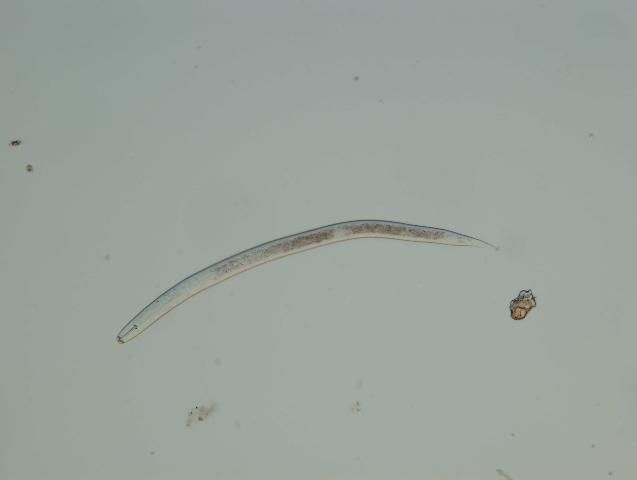
Credit: Zane Grabau, UF/IFAS
Most plant-parasitic nematodes of soybeans complete their life cycle (egg, four pre-adult juvenile stages, egg-producing adult) in about one month, depending on the nematode species and environmental conditions, so nematodes may go through six or more generations in a single growing season. A mature female nematode can produce upwards of hundreds of eggs, depending on the nematode species and environmental conditions, so nematode populations can increase rapidly.
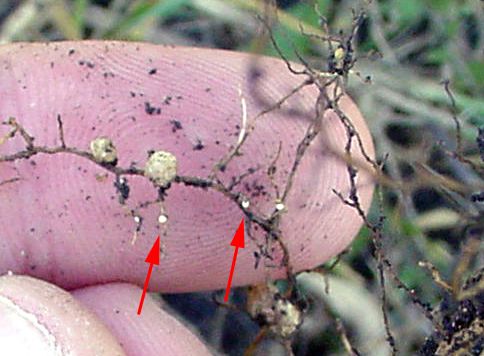
Plant-parasitic nematodes of soybeans spend their entire lives in soil or roots and can be classified by where they reside when feeding, something that is important when sampling for nematodes. Ectoparasites (ecto means outside), including sting nematode, spend their entire lives outside the roots. Only the head end or stylet of an ectoparasite enters the roots when it feeds (Figure 6). (A stylet is a needle-like mouthpart that all plant-parasitic nematodes possess.) Endoparasites (endo means inside) enter the root as a juvenile or adult and remain in the root to feed. Some endoparasites, such as lesion nematode, are mobile while feeding (migratory endoparasite). Other nematodes—such as root-knot, soybean cyst, and reniform nematodes—do not move once they begin feeding (sedentary endoparasite). A portion of the population of endoparasitic nematodes can be found in the soil at any given time because eggs generally hatch in the soil and mobile nematode stages may move freely in soil or from root to root. See the UF/IFAS EDIS publications on sting nematode and reniform nematode for more information about these nematodes.
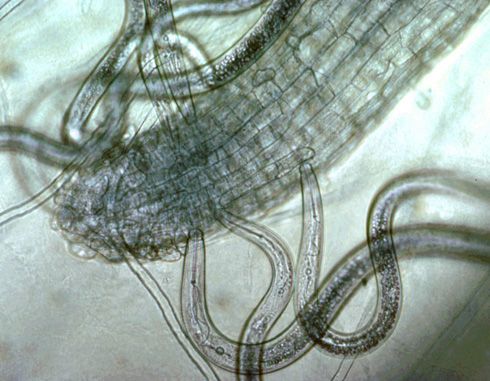
Credit: Ole Becker, University of California, Riverside. Used with permission
Sampling for Nematodes
Determining what nematodes are present in a field and at what densities is helpful for developing a plan for managing nematodes. This can be accomplished by submitting soil or root samples to a professional nematology diagnostic lab such as the University of Florida/IFAS Nematode Assay Laboratory. These samples may be submitted to aid with the diagnosis of disease problems or for advisory service to predict whether or not a potential nematode problem may exist for a future crop. This information allows growers the opportunity to implement management practices to reduce the damage that nematodes may cause.
Detailed information about sampling for nematodes and submitting samples can be found at the UF/IFAS nematode assay laboratory website. When submitting samples for testing for nematodes, either soil or soil and plant root samples should be included. It is suggested to always collect soil because ectoparasitic nematodes can only be recovered from the soil and at least one stage of all endoparasites can be found in soil. It can be useful to collect plant roots in addition to soil when diagnosing crop damage. If the problem is caused by an endoparasitic nematode, then some nematodes will be found inside the roots. Sending pictures of any foliar and root symptoms on the crop is also highly encouraged as it helps with diagnosis and recommendations. Symptoms to look for are described in the "Foliar Symptoms" and "Belowground Symptoms" sections below.
Soil samples for nematodes should be taken to about 12 inches deep. If plants or remnants of plants are still in the field, soil samples should be taken within a few inches of plant stems and intersect plant roots if possible. Because nematode densities vary considerably across a field, about 20 soil cores of about an inch in diameter should be taken from an area of 10 acres or less. Thoroughly mix cores within a single area and submit a 1-pint portion of this mixture for analysis. For larger areas, take multiple, separate samples, making sure to properly label samples. Do not take samples when soil is excessively wet or dry. Store soil samples in closed plastic bags to protect them from drying and keep them cool but not frozen until shipment. When digging root samples, be sure to retain the soil surrounding the roots to slow decay by microbes. Root samples should be collected and stored in a similar manner to soil samples. If samples are intended to diagnose a current crop problem, it may be useful to collect separate samples from the diseased area and a healthy area for comparison.
While samples can be taken at any time of the year, nematode populations fluctuate throughout the year, so timing can be important. In most cases, nematode population densities peak around harvest while plant roots are still in the ground, so that is an ideal time to take routine or predictive samples. When diagnosing crop damage, take samples as soon as you see damage and again around harvest. For questions about sampling for nematodes, contact your local Extension agent, personnel of the Nematode Assay Lab or the author of this paper.
Foliar Symptoms
Symptoms of nematode infection in soybeans can be indistinct and difficult to detect, so routine sampling for nematodes is important. Foliar symptoms of nematode infection may be similar to symptoms of nutrient deficiency or diseases and include stunted, chlorotic (yellowed) plants (Figure 7). These symptoms often occur in oval or irregular patches in the field, corresponding to areas of greater plant-parasitic nematode densities. These patches may surround the initial entry point for the nematodes or correspond to uneven environmental conditions, such as soil type variation.

Credit: Senyu Chen, University of Minnesota
Plants infected with root-knot nematode (Figure 8) may also wilt more than healthy plants during hot, dry conditions, particularly late in the season. Sting nematode infection can cause severe stunting and reduced plant stand, symptoms that tend to appear early in the year. In fields infested with sting nematode, the spatial transition from healthy to diseased plants may be clear and abrupt with apparently healthy plants next to severely stunted ones. Fields recently infected by reniform nematode may exhibit patchy stunting, but this aggressive nematode is often evenly distributed throughout a field after a few years, which leads to uniform stunting that is difficult to detect.

Credit: Mace Bauer, University of Florida
Belowground Signs and Symptoms
Stunted root systems and reduced yield are common, generalized symptoms of plant-parasitic nematode infection. Specific belowground symptoms vary by nematode. Root-knot nematode infection, but not other nematodes, typically causes irregular swellings of the roots called galls (Figure 9). Soybean roots have nitrogen-fixing nodules that should not be confused with galls. Soybean nodules are nearly spherical and attached to the sides of the roots. Nodules can be easily removed from the root while galls cannot be removed without destroying the root.

Credit: Mace Bauer, University of Florida
Galls vary in size based on the severity of infection and may coalesce to cover most of the root surface if nematode densities are very high. These galls are caused by an increase in the size and number of cells triggered by root-knot nematode feeding. Galls contain one or more sedentary adult female root-knot nematodes. These nematodes are contained in the roots and small, about 1 mm in diameter, so it is very difficult to view them in the field. However, with some practice, or the aid of a nematologist, it is possible to excise these pearly white females from the roots and see them—a hand lens will make the examination much easier. Galls may rot as they become entry points for secondary infection by other pathogens.
Soil may cling more easily to roots infected by root-knot or reniform nematodes than uninfected roots. This is because female root-knot and reniform nematodes exude gelatinous egg masses—sac-shaped structures containing hundreds of nematode eggs. Reniform nematode produces these egg masses at the root surface while root-knot nematodes may produce them inside or on the surface of the root. Soil adheres to the roots coated with moist egg masses. Egg masses are brown and about 1 mm or smaller, so they are not readily visible with the naked eye.
Reniform nematode does not induce root galling or any other distinctive below-ground symptoms. Because above-ground symptoms of reniform nematode infection are also indistinct, it is especially important to soil sample regularly for this nematode. Sting nematode causes dark lesions of necrotic root tissue, often near the root tip where this nematode tends to infect. Lateral roots may proliferate above this point, resulting in stunted roots with a hairy or bearded appearance.
Nematode infection, particularly by lesion or sting nematodes, often causes dark lesions of dying tissue on soybean roots. These lesions may join together to cover large portions of the root when infestations are severe. Fungal or bacterial pathogens may also infect dying root tissue. Sting nematodes often infect and kill root tips, which induces lateral root formation above the infection point. This leads to stunted roots with a hairy or bearded appearance.
Belowground symptoms of soybean cyst nematode are usually indistinct. However, with some training, one can recognize the female or cyst stage of soybean cyst nematode on infected soybean roots. After reaching maturity, the back end of female soybean cyst nematodes pushes outside the soybean root and will be just visible with the naked eye or, more easily, with a hand lens. These nematodes are lemon-shaped, range in color from white to yellow to dark brown, and are about the size of the period at the end of this sentence. Do not confuse soybean cyst nematode with the much larger nitrogen-fixing nodules on soybean roots, which are about the size of a bb (Figure 10).
Credit: Greg Tylka, Iowa State University. Used with permission
Management
Once plant-parasitic nematodes infest a field, it is not possible to eradicate them. Rather, the goals are to minimize crop damage by nematodes and keep nematode densities low, ideally at a level where no crop loss occurs. The best way to manage a nematode problem is to use a combination of the most effective and economical practices for the given production situation based on nematode infestation level, other pest problems, available equipment, economics, and other considerations.
Exclusion
Exclusion is taking steps to stop or slow the spread of one or more plant-parasitic nematodes from infested to non-infested fields. Nematodes do not actively migrate from field to field; rather, they are transported in infected soil, water, or plants. Wind and rain move these materials naturally, but they are also carried on field equipment. Besides avoiding intentionally moving soil or plant material, cleaning these materials from field equipment can slow nematode movement. This is particularly important when working both infested and non-infested areas. Nematodes can also be brought in on infected planting material. In general, this is not a large concern for row crops because soybean nematodes do not infect seeds, but closely monitor any crops that are started as transplants.
Crop Rotation
Producers can use crop rotation to reduce nematode densities by growing a crop that the particular nematode cannot reproduce on (a non-host crop) or that the nematode does not reproduce well on (poor host). In the absence of a host to reproduce on, nematode densities decrease as a portion of the nematodes die or are eaten by natural enemies. Weed management is an important supplement to crop rotation because plant-parasitic nematode population densities can be maintained or increased on weedy hosts, including volunteer soybeans, growing in a non-host crop.
A beneficial crop rotation for managing plant-parasitic nematodes varies by the specific nematode(s) infesting a field. As an aid for selecting a useful rotation, the host status of selected crops to some plant-parasitic nematodes of soybeans in Florida is listed in Table 1. On a practical level, soybean is often a secondary crop in Florida that is grown periodically in a rotation, such as with cotton or peanut, so rotation decisions often center around the primary crops rather than soybeans. Because soybeans are a host of many major nematodes of agronomic crops, they are usually not an ideal choice as a secondary crop in a rotation when nematodes are a problem.
Soybean cyst nematode has a very narrow host range that only includes soybeans and closely related legumes, so most rotations where soybeans are not frequently grown will help manage this nematode. In contrast, sting nematode has a very wide host range, and management through crop rotation may not be feasible. The host range of root-knot nematodes is relatively wide and varies by species, so it is important to identify which species is infesting a field. Reniform nematode does poorly on most agronomic crops other than cotton and soybean but does well on most fruit or vegetable crops.
Fallow and Cover Cropping
Offseason periods when a cash crop is not grown should also be considered as part of a crop rotation strategy. These periods are opportunities to reduce or exacerbate nematode problems. Fallowing fields in the offseason can help reduce nematode densities, but only if weeds, including volunteer soybeans, are controlled because many of them serve as hosts for nematodes. However, erosion can be a major problem when fallowing fields, particularly on sandy soils.
If cover crops are grown, choosing a non-host for the nematodes present in a given field is critical for nematode management. Table 1 includes a summary of the host status of selected cover crops for root-knot and sting nematodes. Additionally, some cover crops—such as Brassicas (radish, mustards, etc.)—may have nematicidal properties, directly reducing densities of nematodes although not typically to the same degree as chemical products. The shoots of these crops must be incorporated into the soil to get the maximum benefits for managing nematodes.
Even cover crops with nematicidal properties are hosts of certain nematodes, such as most Brassicas for root-knot nematodes. Nematode reproduction on a good host will generally counteract any nematicidal effects, so host status should be considered for these cover crops as well. For further information on cover cropping for nematode management, see these EDIS publications Cover Crops for Managing Root-Knot Nematodes (https://edis.ifas.ufl.edu/in892), Management of Nematodes and Soil Fertility with Sunn Hemp Cover Crop (https://edis.ifas.ufl.edu/ng043), Management of Nematodes with Cowpea Cover Crops (https://edis.ifas.ufl.edu/in516), and Marigolds (Tagetes spp.) for Nematode Management (https://edis.ifas.ufl.edu/ng045).
Resistant Cultivars
Resistant cultivars prevent or reduce reproduction by one or more types or genera of plant-parasitic nematode. Resistant cultivars can be categorized by their efficacy, which ranges from immune (no individuals of the target nematode can reproduce on the cultivar) to moderately resistant (some individuals can reproduce on the cultivar). Resistant cultivars sustain little or no damage from the target nematode, and growing resistant cultivars reduces field nematode population densities in a similar manner to non-host crops.
Most breeding for resistance in soybeans has focused on soybean cyst nematode, and nearly all commercial cultivars are resistant to this nematode. This resistance greatly diminishes the number of individual soybean cyst nematodes that reproduce on a plant but does not completely stop all reproduction or damage. Most resistance to soybean cyst nematode in commercial cultivars is derived from the same parent line, and an increasing number of nematode populations have adapted to overcome this source of resistance. Some soybean cultivars are also somewhat resistant to root-knot or reniform nematodes, sometimes from the same parent source that imparts resistance to soybean cyst nematode. There are no known commercial soybean cultivars with resistance to sting or lesion nematodes.
Nematicides and Other Commercial Products
Nematicides are chemical products intended to reduce nematode densities. Nematicide application to soybeans is probably not profitable in most cases. This is because soybeans are a relatively low-value crop, and the only products currently labelled for soybeans are fumigants, which are expensive to apply. Nematicide application often only benefits the crop to which it is applied, so growers should not depend on getting any extra value from nematode control in the subsequent crop, either. This is because nematode populations often rebound by the end of the season, even if the nematicide effectively reduces nematode densities early in the season. Growers should test nematode population levels if they are considering nematicide application. Only areas with severe nematode infestation should be targeted.
In addition to nematicides applied to soil or plants, several seed treatments are labeled for use against nematodes. Most of these products are precoated on seeds and may be exclusive to a particular seed brand. Because seed treatments contain small amounts of product that is not distributed widely in the soil, they may protect from some early-season nematode damage, but can only provide modest nematode control and yield increase. Seed treatments are usually relatively low cost, though, so a modest yield increase would provide a return on investment.
Other Practices
Practices that promote plant health may help plants better tolerate nematode infection even if they do not reduce nematode populations. This includes practices such as maintaining soil fertility and tilth, providing adequate water, and managing insects and diseases (see EDIS publication AGR-182, Soybean Production in Florida https://edis.ifas.ufl.edu/ag185). Good knowledge of environmental and biological properties of one's soil can also aid in making good nematode management decisions. As mentioned above, soil type and texture influence where nematode damage is likely to occur.
Some soils keep plant-parasitic nematode densities low despite a susceptible crop. This suppression often develops gradually over a period of time. Practical and tested methods for developing suppressive soils have not been established, but natural predators and pests of nematodes are one cause of nematode-suppressive soils. Recognizing suppressive soil by monitoring nematode densities and cropping history can help cut costs by eliminating unnecessary management practices.
Selected References
Avendano, F., F. Pierce, and H. Melakeberhan. 2004. "Spatial Analysis of Soybean Yield in Relation to soil Texture, Soil Fertility and Soybean Cyst Nematode." Nematology 6:527–545.
Chen, Z., and D. Dickson. 1998. "Review of Pasteuria Penetrans: Biology, Ecology, and Biological Control Potential." Journal of Nematology 30:313–340.
Kinloch, R. A. 1998. "Soybean." p. 317–333. In K.R. Barker, G.A. Pederson, and G.L. Windham (eds.) Plant and nematode interactions. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, WI.
McSorley, R., D. Dickson, and J. DeBrito. 1994. "Host Status of Selected Tropical Rotation Crops to 4 Populations of Root-Knot Nematodes." Nematropica 24:45–53.
Niblack, T. L., A. L. Colgrove, K. Colgrove, and J. P. Bond. 2008. "Shift in Virulence of Soybean Cyst Nematode Is Associated with Use of Resistance from PI 88788" [Online]. Available at http://www.plantmanagementnetwork.org/pub/php/research/2008/virulence/. Plant Health Progress.
Oka, Y. 2010. "Mechanisms of Nematode Suppression by Organic Soil Amendments—A Review." Applied Soil Ecology 44:101–115.
Riggs, R. D., and M. L. Hamblen. 1966. Further Studies on the Host Range of the Soybean Cyst Nematode. Rep. Bulletin 718. Agricultural Experiment Station, Division of Agriculture, University of Arkansas, Fayetteville, AK.
Robbins, R., E. Shipe, L. Rakes, L. Jackson, E. Gbur, and D. Dombek. 2002. "Host suitability of Soybean Cultivars and Breeding Lines to Reniform Nematode in Tests Conducted in 2001." Journal of Nematology 34:378–383.
Robinson, A., R. Inserra, E. Caswell-Chen, N. Vovlas, and A. Troccoli. 1997. "Rotylenchulus Species: Identification, Distribution, Host Ranges, and Crop Plant Resistance." Nematropica 27:127–180.
Thomas, S., J. Schroeder, and L. Murray. 2005. "The Role of Weeds in Nematode Management." Weed Science 53:923–928.
Wang, K., R. McSorley, R. N. Gallaher, and N. Kokalis-Burelle. 2008. "Cover Crops and Organic Mulches for Nematode, Weed and Plant Health Management." Nematology 10:231–242.



