Tospovirus-Resistant Tomato Varieties for Southern Florida
Situation
Tospoviruses are plant-infecting viruses named after Tomato spotted wilt virus (TSWV), the first member of this group. Currently, 11 tospovirus species are recognized, with additional species proposed. Three tospovirus species are important to Florida tomato production: TSWV, Tomato chlorotic spot virus (TCSV), and Groundnut ringspot virus (GRSV). In northern Florida, TSWV outbreaks have occurred at epidemic levels since the late 1980s, but TSWV has only sporadically infected tomato plants in central and southern Florida. TCSV and GRSV, on the other hand, emerged in southern Florida and were first detected in 2012 and 2009, respectively (Londoño et al. 2012; Webster et al. 2010). TCSV and GRSV have significantly impacted tomato production in southern Florida (Webster et al. 2015), and TCSV is currently the most widely prevalent tospovirus in this region. The symptoms of TSWV, TCSV, and GRSV are nearly identical and cannot be easily distinguished by eye. Some of the common symptoms are necrotic orchlorotic yellow rings on leaves, stunting, and wilting (Figure 1a). Tospovirus infection may also result in spotted fruit; this can render fruit unmarketable (Figure 1b).

Credit: (a) Rebecca Wente, GCREC; (b) Scott Adkins, USDA
Tospovirus Vectors
Tospoviruses are transmitted exclusively by thrips (Family: Thripidae) (Riley et al. 2011), which are very small, slender insects with fringed wings. A key vector of tospoviruses, the western flower thrips (Frankliniella occidentalis), was introduced in northern Florida in the 1980s and is now a major pest of tomatoes. The common blossom thrips (Frankliniella schultzei) is also an efficient tospovirus vector and has become common in southern Florida where TCSV and GRSV outbreaks have emerged (Webster et al. 2015). The western flower thrips has developed resistance to numerous classes of insecticides, including carbamates, pyrethroids, organochlorines, neonicotinoids, avermectins, phenylpyrazoles, and spinosyns (Bielza 2008; Bielza et al. 2009; Bielza et al. 2007; Chen et al. 2011; Espinosa et al. 2002; Espinosa et al. 2005; Gao et al. 2012; Guillén and Bielza 2013; Puinean et al. 2013; Zhao et al. 1995). Effective disease control requires an integrated pest management strategy against the tospoviruses, as well as the thrips vectors. Effective tactics in an integrated pest management program of tomato include proper scouting and use of economic thresholds, UV-reflective mulches and barriers, insecticides, and resistant cultivars. For more detailed information regarding thrips and tospovirus control, see EDIS publication ENY859, "Managing Thrips and Tospoviruses in Tomato". It should be emphasized that insecticides targeted at adult thrips vectors (even when efficacious) will not prevent primary disease spread, and that insecticides targeted against the larvae can be important in preventing secondary disease spread. Resistant cultivars are the most effective tactic for preventing disease losses in tomato.
Varietal Resistance
Selection and planting of virus-resistant tomato varieties is often an effective approach to reduce the economic impact of tospoviruses. Sw-5 is a major gene for TSWV resistance that also confers resistance to TCSV and GRSV (Boiteux and Giordano 1993). Because tospoviruses were not a significant problem in southern Florida before 2009, resistance was not a major emphasis for this production area. Additionally, whereas numerous Sw-5-containing varieties have been developed for production in northern Florida and other parts of the southeastern US, very few of these have been tested for adaptability in southern Florida. In light of the current situation, it is important to both 1) determine the adaptability of existing Sw-5 commercial varieties for production in the Homestead area of Dade County, and 2) develop new resistant varieties bred specifically for southern Florida's growing conditions. The purpose of this article is to describe the performance of several tospovirus-resistant tomato hybrids (commercial or pre-commercial) based on grower trials in the Homestead production area.
Field Trials
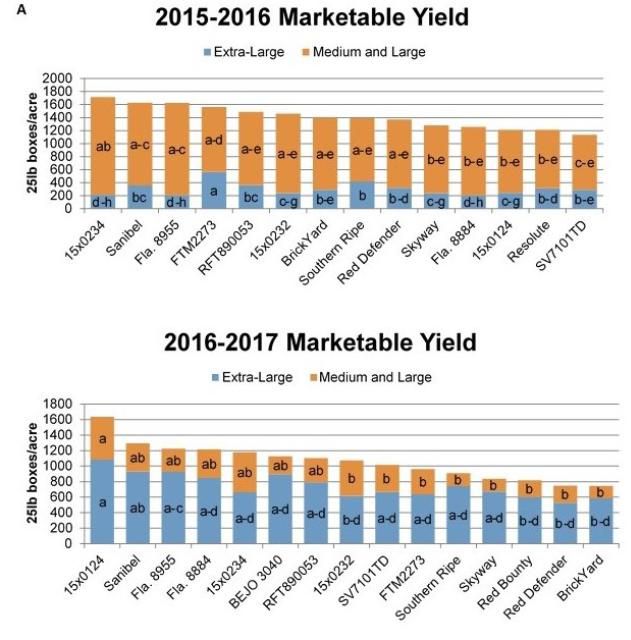
For most crops, growers seldom adopt a new, disease-resistant variety unless its yield and horticultural traits are comparable to currently grown, susceptible varieties. Tomato is no different. In the Homestead production area, 'Sanibel' is widely grown and serves as a benchmark 3 for yield. This tospovirus-susceptible variety has jointless pedicels (a desirable trait that improves stem-free harvest of fruit), a strong vine, good bacterial spot tolerance, and fair resistance to the fruit disorder graywall. Multiple large-fruited, tospovirus-resistant hybrids were evaluated under commercial settings in the Homestead production area during the winter 2015–16 and 2016–17 seasons (Table 1).
Figure 2 depicts yield trends for some of the hybrids that were tested in these trials. Results from additional hybrid tests are described by Hutton et al. (2016). During both seasons, most varieties tested did not differ from 'Sanibel' for total marketable yield, indicating these hybrids may be suitable tospovirus-resistant variety alternatives for growers in Homestead to consider (Fig. 2a, 2b). Extra-large fruit yields were generally low for all hybrids in the 2015–2016 season, a trend consistent with grower reports during that timeframe. Many of the varieties trialed had extra-large yields similar to 'Sanibel'. However, some varieties had lower yields of extra-large fruit compared with 'Sanibel,' and growers should pay close attention to this if they choose to trial any of these varieties. Because graywall can be a problem for tomatoes produced in the winter, resistance to this disorder is important. Only one of the varieties trialed ('Quincy') showed graywall susceptibility and therefore may not be a good variety choice for southern Florida. Disease resistance packages varied among the hybrids trialed. Although resistance to Fusarium crown rot and Fusarium wilt are not as important in the Homestead production area, Tomato yellow leaf curl virus (TYLCV) can be a significant problem, and those varieties which also carry resistance or tolerance to this virus may be of particular interest to growers. Although none of the commercially available tospovirus-resistant varieties have jointless pedicels, some of the new hybrids being developed in the UF/IFAS tomato breeding program carry this trait and may have potential for future release. These results provide practical information for growers interested in tospovirus-resistant variety alternatives for southern Florida.
Acknowledgements
We sincerely thank DiMare Fresh and Circle D Farms for their generous support of this work. Funding for this research was provided in part by the Florida Department of Agriculture and Consumer Services Specialty Crop Block Grants 22901 and 24049.
Works Cited
Bielza, P. 2008. "Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis." Pest Mgt. Sci. 64:1131–1138.
Bielza, P., Fernández, E., Grávalos, C., and Abellán, J. 2009. "Carbamates synergize the toxicity of acrinathrin in resistant western flower thrips (Thysanoptera: Thripidae)." J. Econ. Entomol. 102:39 –397.
Bielza, P., Quinto, V., Fernandez, E., Gravalos, C., and Contreras, J. 2007. "Genetics of spinosad resistance in Frankliniella occidentalis (Thysanoptera: Thripidae)." J. Econ. Entomol. 100:916–920.
Boiteux, L.S. and Giordano, L.d.B. 1993. "Genetic basis of resistance against two Tospovirus species in tomato (Lycopersicon esculentum)." Euphytica 71:151–154.
Chen, X., Yuan, L., Du, Y., Zhang, Y., and Wang, J. 2011. "Cross-resistance and biochemical mechanisms of abamectin resistance in the western flower thrips, Frankliniella occidentalis." Pesticide Biochem. Physiol. 101:34–38.
Espinosa, P.J., Bielza, P., Contreras, J., and Lacasa, A. 2002. "Insecticide resistance in field populations of Frankliniella occidentalis (Pergande) in Murcia (south-east Spain)." Pest Mgt. Sci. 58:967–971.
Espinosa, P.J., Contreras, J., Quinto, V., Grávalos, C., Fernández, E., and Bielza, P. 2005. "Metabolic mechanisms of insecticide resistance in the western flower thrips, Frankliniella occidentalis (Pergande)." Pest Mgt. Sci. 61:1009–1015.
Gao, Y., Lei, Z., and Reitz, S.R. 2012. "Western flower thrips resistance to insecticides: detection, mechanisms and management strategies." Pest Mgt. Sci. 68:1111–1121.
Guillén, J. and Bielza, P. 2013. "Thiamethoxam acts as a target‐site synergist of spinosad in resistant strains of Frankliniella occidentalis." Pest Mgt. Sci. 69:188–194.
Hutton, S.F., Adkins, S., Funderburk, J.E., and Turechek, W.W. 2016. "Tospo-Resistant Variety Outlook for South Florida." Proc. Florida Tomato Institute. 532:6–7.
Londoño, A., Capobianco, H., Zhang, S., and Polston, J.E. 2012. "First record of Tomato chlorotic spot virus in the USA." Trop. Plant Pathol. 37:333–338.
Puinean, A.M., Lansdell, S.J., Collins, T., Bielza, P., and Millar, N.S. 2013. "A nicotinic acetylcholine receptor transmembrane point mutation (G275E) associated with resistance to spinosad in Frankliniella occidentalis." J. Neurochemistry 124:590–601.
Riley, D.G., Joseph, S.V., Srinivasan, R., and Diffie, S. 2011. "Thrips vectors of Tospoviruses." J. Integrated Pest Mgt. 2:I1–I10.
Webster, C.G., Frantz, G., Reitz, S.R., Funderburk, J.E., Mellinger, H.C., McAvoy, E., Turechek, W.W., Marshall, S.H., Tantiwanich, Y., and McGrath, M.T. 2015. "Emergence of Groundnut ringspot virus and Tomato chlorotic spot virus in vegetables in Florida and the southeastern United States." Phytopathology 105:388–398.
Webster, C.G., Perry, K.L., Lu, X., Horsman, L., Frantz, G., Mellinger, C., and Adkins, S. 2010. "First report of Groundnut ringspot virus infecting tomato in south Florida." Plant Health Prog. 710:1094.
Zhao, G., Liu, W., Brown, J.M., and Knowles, C.O. 1995. "Insecticide resistance in field and laboratory strains of western flower thrips (Thysanoptera: Thripidae)." J. Econ. Entomol. 88:1164–1170.
Tables
Tospovirus-resistant and susceptible varieties were tested in Homestead, FL. Some varieties are commercially available, while others are experimental hybrids being developed by seed companies or by the UF/IFAS Tomato Breeding Program. Key to Disease Resistance: TSWV = Tomato spotted wilt virus, Fol = Fusarium Wilt: three races, For = Fusarium crown and root rot, Va/Vd = Verticillium wilt, Ma/Mi/Mj = Southern root-knot nematode, TYLCV = Tomato yellow leaf curl virus: (Tol = tolerance).


