Identification and Treatment of European Foulbrood in Honey Bee Colonies
Introduction
European foulbrood (Melissococcus plutonius), often referred to simply as EFB, is a bacterial disease that affects western honey bee (Apis mellifera) brood. European foulbrood is found on all continents where Apis mellifera is kept, making it a concern to beekeepers everywhere (Ellis 2008). European foulbrood can be confused with another bacterial disease, American foulbrood (Paenibacillus larvae), commonly referred to as AFB, because they have similar life cycles and colonies infected by both show comparable signs of disease. However, European foulbrood is far less serious than American foulbrood because Melissococcus plutonius does not form spores, while Paenibacillus larvae does. Consequently, European foulbrood can be treated, but American foulbrood cannot. Beekeepers must be able to identify European foulbrood and differentiate between it and American foulbrood to manage their colonies effectively and prevent the spread of both diseases (Table 1).
Life Cycle
A larva younger than 24 hours old can be infected with European foulbrood (Ellis 2008) when they are supplied with food contaminated with the bacteria by nurse bees. European foulbrood multiplies in the larval gut and competes for nutrition with infected larva. The larva's appetite increases as competition between the bacteria and infected larva increases. In strong colonies, nurse bees will remove abnormally hungry larvae, and thereby remove the disease from the nest. European foulbrood will not be a major problem for the colony as long as bees are able to remove dead and infected larvae in a timely manner. Strong colonies can control European foulbrood. However, the bacteria can remain in brood cells or in feces excreted by infected larvae even after the larvae have been removed from their cells by adult bees (Delaplane 2006). Therefore, nurse bees can pick up and transfer the disease from infected to healthy larvae in the colony when feeding and cleaning brood.
Signs of Disease
Healthy larvae appear plump and pearly white. They lie in the backs of their cells in the shape of a letter "C" (Figure 1). Diseased larvae often appear melted, shrunken, deflated, rubbery, or dehydrated. They also may be white, yellow, or yellowish-brown in color, with their tracheal systems visible through their integuments. Additionally, diseased larvae may twist or curl upward in the uncapped cell (Figure 2).

Credit: Waugsberg, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=2009806
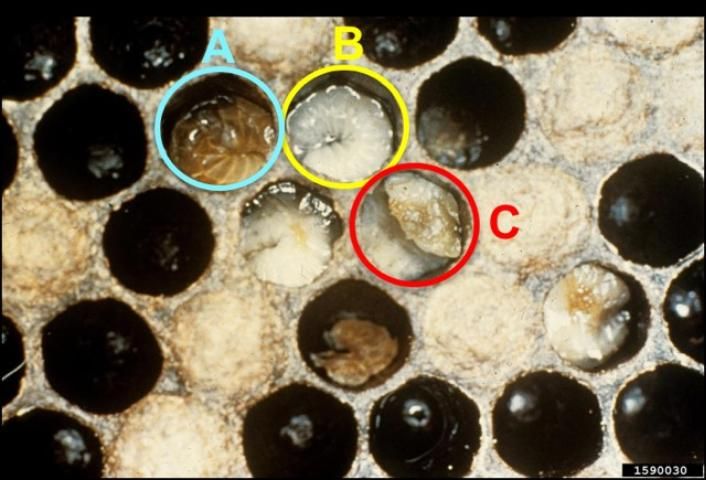
Credit: Georgia Department of Agriculture, CC BY-NC 3.0 https://www.invasive.org/browse/detail.cfm?imgnum=1590030
A brood comb infected with European foulbrood may appear spotty with intermittent capped and empty cells scattered throughout the comb (Figure 3). This spotty pattern occurs when some larvae survive and are capped while others die from European foulbrood and are removed from their cells by their adult sisters. A spotty brood pattern is not associated exclusively with European foulbrood, as other conditions can cause a similar brood pattern. However, it can be an additional indicator of European foulbrood infection when observed with larvae showing other signs of disease.

Credit: UF/IFAS Honey Bee Lab
The name "foulbrood" is used to describe the bad smell associated with colonies infected with European foulbrood or American foulbrood, though not all diseased colonies have a bad odor. A beekeeper may smell a sour, rank, or rotting smell when they open the hive if there is a high level of infection. For European foulbrood, the smell may result from secondary bacterial infections that co-occur with Melissococcus plutonius. Some describe colonies infected with European foulbrood as having a sour smell while those infected with American foulbrood smell sulfurous (Delaplane 2006). The smell of a colony cannot be used to differentiate between a European foulbrood or American foulbrood infection because the smell can be ambiguous and will vary based on the degree of infection.
Many beekeepers use the rope test to attempt to differentiate between European foulbrood and American foulbrood. The rope test is performed by sticking a small twig, match stick, etc. into a single brood cell containing a larva or pupa showing signs of disease. The contents of the cell are stirred with the twig, and the twig is then withdrawn slowly from the cell. The contents of the cell will rope (American foulbrood) or not rope (European foulbrood), depending on the species of bacteria that killed the developing bee (Delaplane 2006). The cell contents are considered ropey if they strand out 1 or more inches (2.54 cm) (Figure 4). This test is useful for field diagnostic purposes when coupled with other signs of disease.

Credit: M. Bammer, UF/IFAS Honey Bee Lab
Diagnostic test kits can be purchased and used in the field to detect European foulbrood and/or American foulbrood quickly and accurately. The Vita European foulbrood Diagnostic Test Kit comes with full instructions and everything one needs to perform the test. The kits are specific to European foulbrood or American foulbrood. Be sure to purchase and use the kit for the bacterial disease for which you intend to test.
Necessary supplies (provided in the kit; 1 kit per colony tested).
- 1 spatula
- 1 extraction bottle (screw-cap container filled with extraction fluid and ball bearings).
- 1 pipette
- 1 sample well
Detailed instructions for Vita European foulbrood diagnostic kit (Figure 5):

Credit: C. Mueller, UF/IFAS Honey Bee Lab
- Label the extraction bottle with the date and name/label of the colony to be sampled.
- Remove the lid from the extraction bottle.
- Collect larvae showing signs of infection with European foulbrood from the combs with the provided spatula (Figure 6).
- Place the larvae into the extraction fluid with the spatula (Figure 7).
- Replace the lid of the extraction bottle and shake vigorously for 20 seconds (Figure 8).
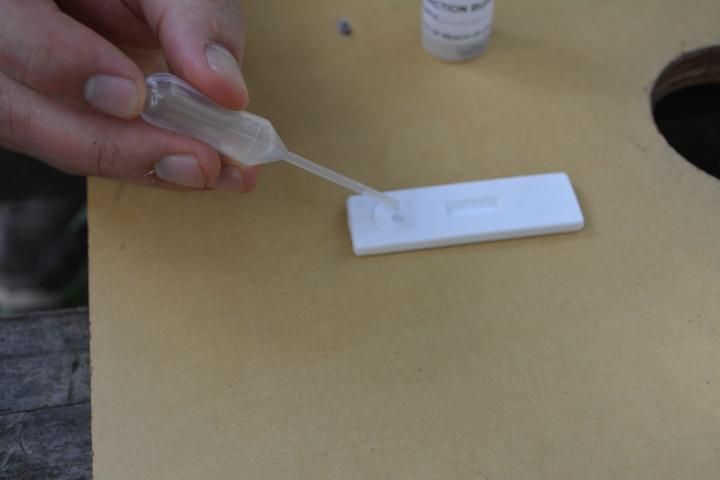
- Remove the lid of the extraction bottle, and use the supplied pipette to remove a liquid sample from the bottle (Figure 9).
- Squeeze two to three drops into the sample well (the circular indentation in the plastic) (Figure 10).
- Keep the sample well horizontal and wait three minutes.
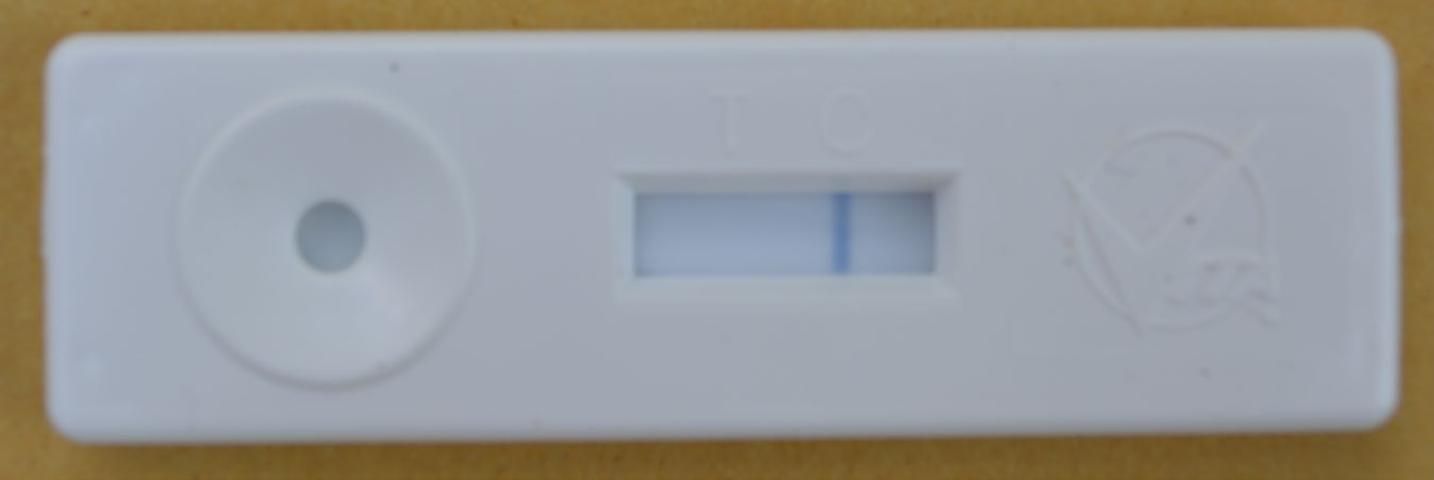
- Read your results. The test is negative if only one blue line above the C is visible (Figure 11). The sample is positive for European foulbrood if there are two lines present, one above the C and another above the T.
- If no lines are present in the sample well, the test failed, and you will have to re-test.

Credit: C. Mueller, UF/IFAS Honey Bee Lab
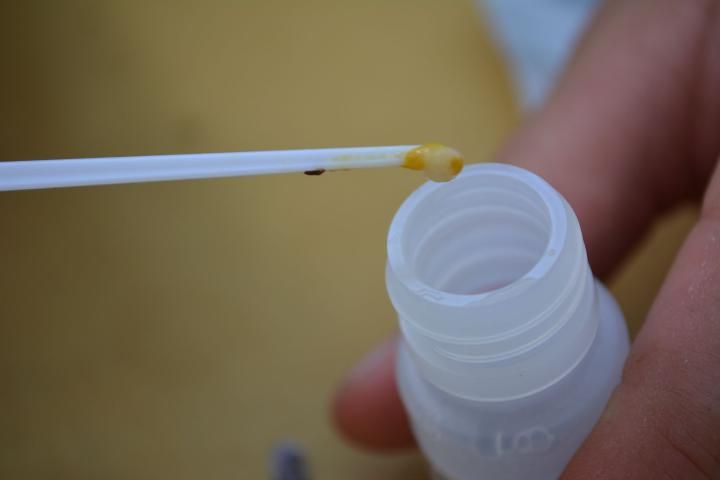
Credit: C. Mueller, UF/IFAS Honey Bee Lab

Credit: C. Mueller, UF/IFAS Honey Bee Lab

Credit: C. Mueller, UF/IFAS Honey Bee Lab
Credit: C. Mueller, UF/IFAS Honey Bee Lab
Credit: C. Mueller, UF/IFAS Honey Bee Lab
While these diagnostic kits are very accurate, it is possible for false negatives or false positives to occur (Tomkies et al. 2009). A false negative may occur in any diagnostic test if the sample selected from an otherwise infected hive did not happen to contain the targeted bacteria. False positives, while extremely rare, have been reported. In either case, multiple tests of the same colony in question may be performed for confirmation.
Preventing European Foulbrood
It is important that beekeepers maintain proper sanitation practices to prevent the spread of European foulbrood. Sanitize equipment properly before storing it, reusing it, or moving it from one colony to another. First, wash hive tools, smokers, and frames with soapy water, and follow by disinfecting tools and equipment by soaking in a 1:5 bleach solution (1 part bleach: 5 parts water by volume).
It is best to start with new equipment and tools that have not been exposed to European foulbrood when starting with new colonies. Starting colonies from packages may reduce the risk of introducing contaminated equipment into your apiary. Inspect all combs for signs of diseased brood when purchasing nucs or full-size colonies. Do not purchase suspect colonies.
Hygienic stocks of bees can be effective at reducing the incidence of European foulbrood infections. There are no lines or breeds of bees that are resistant to European foulbrood; however, hygienic queens produce worker offspring that remove diseased larvae from the hive, ultimately mitigating the effects of infection from European foulbrood. Hygienic bees also help control other pests and diseases, such as Varroa destructor, sacbrood virus, and chalkbrood (Ascophaera apis).
Antibiotics can be applied to colonies prophylactically to prevent European foulbrood. Beekeepers need a prescription or Veterinary Feed Directive from a licensed veterinarian to obtain appropriate antibiotic treatments. Prescriptions are good for one year, and a Veterinary Feed Directive is good for six months; you must keep records of Veterinary Feed Directive orders for two years. Once you have your prescription or Veterinary Feed Directive, you will be able to purchase an antibiotic through a beekeeping equipment company (if they re-registered with the FDA Center for Veterinary Medicine), a pharmacist, or a veterinary clinic (Bammer 2016). Use the prescribed antibiotic in accordance with the label.
Treatment after Diagnosis
Antibiotics are used prophylactically to prevent and to treat European foulbrood after signs of the disease are visible. Oxytetracycline (Terramycin) is the only antibiotic approved by the US Food and Drug Administration to treat European foulbrood. There are concerns that European foulbrood could become resistant to Terramycin (Wilson and Skinner 2009), but at present, published reports of European foulbrood resistance to Terramycin do not exist. Terramycin is typically applied in biannual treatments (in the spring and fall) if used prophylactically. Terramycin should not be applied to colonies before a nectar flow. Beekeepers should always follow the product label when treating colonies for European foulbrood.
There are several non-chemical methods that can be used to treat European foulbrood infections. For instance, adding additional frames with young and mature brood from a non-infected colony to an infected one can help the infected colony by increasing competition between healthy and sick brood. Nurse bees will have more brood to tend and, as a result, sick brood receive less food. The sick brood will die and be eliminated from the hive (Delaplane 2006). The elimination of sick larvae reduces the amount of European foulbrood in the hive.
Another potential European foulbrood remedy is to simulate a nectar flow by feeding bees a 1:1 sugar syrup, (1 part water: 1 part sugar by volume). Feeding bees sugar syrup stimulates the production of new brood in a colony. Thus, more workers will be available to forage. The addition of brood will result in less care for individual larvae, allowing healthy larvae to outcompete sick larvae.
Finally, the European foulbrood cycle can be disrupted using the shook swarm method (Wilson 2009). In this method, you will move adult bees from the infected hive equipment into new/disease-free equipment.
Supplies needed to implement a shook swarm:
- 1 new or disease-free deep super or brood box that accommodates 8 or 10 frames (based on whatever size the beekeeper uses)
- 8 or 10 (depending on your equipment) new or disease-free frames with foundation or pulled comb
- 1 new or disease-free bottom board
- 1 new or disease-free queen excluder
- 1 queen cage
- 1 new or disease-free migratory cover
- 1:1 sugar syrup for feeding
- antibiotics
Detailed Instructions
- Set up a new or disease-free hive. The hive should contain frames of foundation or disease-free combs.
- Remove 3 to 4 frames from the center of this hive and set them aside.
- Find and cage the queen in the infected colony. Place the caged queen in a safe place out of the sun.
- Remove one frame from the infected hive and shake the bees from it into the new hive.
- Discard the infected frame.
- Repeat steps 4 and 5 with all remaining frames from the brood nest of the original hive.
- Replace the frames set aside in step 2 into the new hive once all of the bees have been shaken into it.
- Release the queen into the brood chamber of the new hive.
- Place the queen excluder on the new hive.
- Repeat steps 2 to 7 for all supers the old, infected hive has.
- Treat colony for European foulbrood with antibiotics. Follow the label instructions.
Selected Reference
Bammer, M. 2016. "New Year, new rule: A look at the FDA ruling prescriptions for honey bees." https://blogs.ifas.ufl.edu/entnemdept/2016/12/23/new-year-new-rule-look-fda-ruling-prescriptions-honey-bees/ (Accessed 23 September 2019).
Delaplane, K. 2006. Honey Bees and Beekeeping: A year in the life of an apiary. Athens, GA, USA: The University of Georgia.
Ellis, Jamie. 2008. "A video field guide to beekeeping [Episode 5]. American and European foulbrood." http://articles.extension.org/pages/25099/university-of-florida-bee-disease-videos (Accessed 1 July 2018).
Tomkies, V., J. Flint, G. Johnson, W. Ruth, S. Wilkins, C. Danks, et al., 2009. "Development and validation of a novel field test kit for European foulbrood." Apidologie 40: 63–72.
Wilson, M. 2009. "Shook swarm and OTC antibiotics for European foulbrood control." http://articles.extension.org/pages/23697/shook-swarm-and-otc-antibiotics-for-european-foulbrood-control (Accessed 23 September 2019).
Wilson, M., and J. Skinner. 2009. "European foulbrood: a bacterial disease affecting honey bee brood." https://bee-health.extension.org/european-foulbrood-a-bacterial-disease-affecting-honey-bee-brood/ (Accessed 9 February 2023).




