Caribbean Spiny Lobster
General Description
The Caribbean Spiny Lobster, Panulirus argus, is a member of the family of spiny lobsters, Palinuridae. The animal has a cylindrical carapace, which is covered with forward-projecting spines, and two prominent rostral horns which extend over the eyes. The most recognizable characteristic of the species is the pair of long, whip-like antennae, which are covered in short 1–2 mm spines. The tail is smooth, and the tail fan is composed of a central telson bordered by a pair of uropods on either side. Adult coloration varies from a green and brown to deep red and black dorsal carapace and tail with light gray to tan sides and ventral surface. Individuals also have pronounced white to yellow ocelli on the second and sixth tail segments.
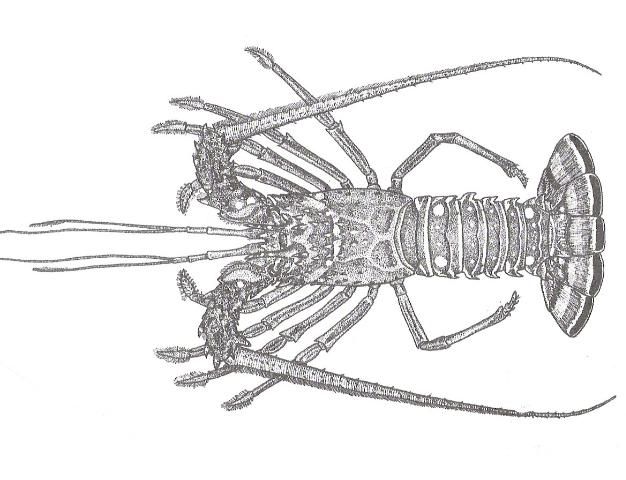
Credit: FAO, Rome, Italy. 1978.
The spiny lobster inhabits coastal waters and shallow continental shelf waters of the Atlantic seaboard and portions of the Gulf of Mexico. Its range extends from North Carolina southward to Brazil, including Bermuda and the Gulf of Mexico (Marx and Herrnkind 1986). The spiny lobster inhabits a variety of marine habitats depending on its stage in life; these include the open ocean (pelagic), shallow coastal areas (hard bottom and mangrove habitat), and near-shore reef zones.
Driven by high market value and limited availability, the development of both experimental and commercial-scale spiny lobster aquaculture operations are ongoing worldwide. There has been considerable interest in developing culture technologies for the 47 species (Lipcius and Eggleston 2000) in this family. Past studies focusing on the economic feasibility of palinurid lobsters indicate that the tropical species of this family shows the most promise for commercial production due to its relatively fast growth rates and high market value (Jeffs 2003). The species of greatest interest and demand for information on culture techniques in Florida is the Caribbean spiny lobster, Panulirus argus.
Natural History
There are five major phases in the life cycle of spiny lobsters: (1) oceanic planktonic phyllosoma larvae, (2) swimming postlarval puerulus, (3) early benthic algal-dwelling juvenile (< 20 mm carapace length, CL), (4) later crevice-dwelling juveniles (20-65 mm CL), and (5) adults (Marx and Herrnkind 1986).
In Florida, spawning occurs from April through October when water temperatures exceed 23°C (73°F). Larval biology and ecology of this species is poorly understood. Larval development is very protracted, or long; the phyllosoma larvae go through 11 morphological stages during approximately 12 months, prior to metamorphosis. Phyllosoma larvae use their long, fan-like feeding appendages to feed on a wide variety of plankton.
The phyllosoma larval stage ends in a metamorphosis to a short benthic settling stage known as a puerulus. During this stage the pueruli do not feed as they migrate from the open ocean to shallow, in-shore mangrove/macro-algal habitats and molt and metamorphose into juvenile lobsters. Juvenile and adult lobsters are opportunistic nocturnal foragers. Their diet is comprised of a variety of invertebrates including gastropods, bivalves, crustaceans, and echinoderms (Marx and Herrnkind 1986). In general, spiny lobsters reach three inches in carapace length between 1.5 to 2 years after settlement. This is the legal size for recreational and commercial catch in Florida. The carapace, which consists of a fused head and thorax, is the exoskeleton section between the head and tail sections and is measured from the forward edge between the rostral horns, excluding any soft tissue, and back to the rear edge before the start of the tail section. For further information on harvesting laws and measuring the carapace, see the Florida Fish and Wildlife Conservation Commission website.
Culture Methods
Broodstock
P. argus in Florida typically mates between March and June. During mating, the male passes a spermatophore called a "tarspot" to the female, and it attaches to the ventral side of her abdomen. The eggs are released from the female's gonopore, which is located at the base of the thirds walking leg. The female ruptures the spermataphore using her fifth walking leg, and the eggs are fertilized as they pass over the spermataphore. The fertilized eggs are then attached to, and carried externally on, the ventral side of the tail where they become darker as they develop. Spawning in Florida typically occurs from April through October when water temperatures exceed 23°C (73°F). Reproductive maturity of P. argus in the wild is believed to be at about 3 to 4 years of age, including the larval stage. Fecundity varies directly with the size of the animal, and ranges from 250,000 to more than one million eggs per spawn. Egg size averages 0.5 mm in diameter.
Hatchery
The length of the phyllosoma larval stage in the wild is unknown. However, in an aquaculture setting while providing what is believed to be the optimal quantity and quality of natural food the larval phase lasts about 12 months. Currently, all attempts to decrease this time through different management strategies have failed. Thus, due to the complex and long larval cycle, hatchery production of P. argus juveniles does not appear to be viable economically at present. For more information on the phyllosoma larval stage, see Kittaka (1997), and Jeffs and Davis (2003).
Nursery
Acquiring pueruli from aquaculture has been shown to be possible but given the length of the larval stage it is not economically feasible. However, collection of wild-caught pueruli has been studied and with refinement of collection techniques could become an economically viable approach for commercial aquaculture. Due to high mortality of pueruli lobster in the wild, an estimated 95% are lost to predation of pre-settlement pueruli and this is believed to have no effect on recruitment rates of juvenile lobster (Butler and Herrnkind 1989). Various devices, which can be inexpensively constructed, have effectively harvested pueruli from the wild. One simple design, a Witham collector (Witham et al. 1968), is made of a pvc frame and a substrate material which dangles into the water column. It is estimated that commercial-scale collection operations using Witham collectors or similar devices could be expected to provide large quantities of pueruli at an estimated cost of $0.05 to $0.30 per seed lobster (Jeffs and Davis 2003). This estimated cost is dependent on the number of pueruli available at a given time and region and thus may vary greatly.
There are currently no land-based, lobster grow-out facilities in Florida or the Caribbean region. Small-scale experimental grow-out of lobsters has been attempted with some level of success in various marine culture systems including flow-through, semi-recirculation, and full recirculation (Ting 1973; Witham 1973; Lellis 1991; Sjoken 1999; Sharp et al. 2000). A wide variety of enclosures has been used with and without shelters including glass aquaria, as well as fiberglass, plastic, and concrete tanks, with and without shelters. The roles of tank design and holding conditions on growout have not been directly assessed.
Growout
Sea-cage culture of spiny lobster is a method which has shown considerable promise in other areas of the world, but it has received little research interest in Florida to this point. Assad et al. (1996) stocked a 500 m2 cage with 15,000 wild-caught juvenile lobsters and recorded a growth rate of 1g/day tail weight after 60 days of holding. Sea-floor cages (3x3x1 m) have been used for holding and feeding wild-caught juveniles in Mexico (Lozano-Alvarez 1996), and other studies have attempted to increase the biomass of wild-caught lobsters with mixed results (Creswell 1984; Brown et al 1999; Jeffs and James 2001). These small cages are commonly used in Vietnam and other countries in the Asian region for growout or "fattening" of benthic juvenile spiny lobster species native to that region.
Today there are no commercially effective formulated diets for spiny lobster culture of any life stage. There are no spiny lobster reference diets produced by researchers with which to compare different diets. Initial studies indicate that spiny lobster juveniles and adults are not readily weaned to artificial diets and typically local mollusks and oily fish serve as the best known food source. Since live, fresh, or frozen seafood is expensive to collect, transport, and store, a shelf-stable formulated feed is essential for the spiny lobster aquaculture industry to evolve. Recent experiments with P. argus in Florida revealed that feeding juvenile spiny lobsters rations of frozen clams, shrimp, squid, and oysters at 100% of their body weight once daily at the onset of dusk resulted in significantly better growth than those fed 50% of their body weight twice daily (Cox and Davis 2006).
Market
There is strong worldwide demand for species belonging to this family of spiny lobsters. Total world landings exceed 75,000 metric tons, with a dockside value of approximately $500 million dollars (Jeffs 2003). The average yearly domestic commercial harvest of spiny lobsters from 2000 to 2004 was in excess of 4.5 million pounds. The fishery had an average dockside value of $21.5 million dollars (NMFS 2005). While the natural range of the Caribbean spiny lobster is extensive, the United States' commercial harvest is confined to the state of Florida.
Disease
Few diseases have been reported with spiny lobsters. Bacterial diseases common to other crustaceans including white spot, vibrio, and chitinoclastic bacteria which cause lesions on the tail have been reported, however, all of these only occurred following stressful events. The disease, gaffkemia, a common problem in clawed lobsters, is believed to occur naturally but has not been reported in wild and cultured spiny lobsters. Some fungal infections, on the carapace, gills, and on larvae, have been reported in cultured spiny lobsters and were identified as Fusarium sp. and Legenidium sp. common to crustaceans. Compared to other cultured crustaceans, few diseases of spiny lobsters have been reported, although disease issues may become more apparent as a greater number of spiny lobsters are maintained in culture facilities.
Few parasites have been reported in wild spiny lobsters. However, recently the first naturally occurring virus found in any lobster was discovered in the Caribbean spiny lobster (Shields and Behringer 2004). This virus, PaV1, has an estimated overall prevalence of 5% in the Florida Keys, but may be locally higher and is found in greatest concentrations among the smallest juveniles (16% in juveniles < 20 mm CL). There have also been reports of PaV1 infections throughout the Caribbean including the US Virgin Islands, Mexico, and Belize. This is especially important for aquaculture operations where small lobsters would be held at high densities. Currently, UV sterilization appears to be an effective means to control the spread of PaV1.
Summary of Aquaculture Potential in Florida
Spiny lobster P. argus has a rapid growth rate, high demand and market price, and can likely be cultured in a wide variety of culture systems. Therefore, aquaculture of this species may have potential as a commercial enterprise in the future. Before recommendations can be made on specific aquaculture protocol, further research is necessary to develop economically viable, land-based and open-ocean cage systems. Important research and development that still are lacking include improvement of technology for capture of wild pueruli, design and testing of various culture systems, determination of optimal management practices, and development of acceptable formulated feeds.
In summation, P. argus aquaculture is in the research stage in the United States. Further research is needed to define economically viable culture techniques on a small scale before being tested and verified on a commercial scale in Florida.
References and Recommended Additional Reading
Assad, L.T., D.S. Gondim, and M. Ogawa. 1996. The growout of spiny lobster juveniles in marine cages. Arquivos Cienias do Mar, Fortaleza 30: 13–19.
Brown, P., R. Leader, S. Jones, and W. Key. 1999. Use of New Water-stable Feed for Culture of the Spiny Lobster (Panulirus argus). Purdue Experimental Station Technical Publication. 1: 1–8.
Butler, M.J. and W.F. Herrnkind. 1989. Are artificial Witham surface collectors adequate indicators of Caribbean spiny lobster, Panulirus argus, recruitment. Proceedings of the Gulf and Caribbean Fisheries Institute. 42: 135–136.
Cox, S.L. and M. Davis. 2006. The effect of feeding frequency and ration on growth of juvenile spiny lobster, Panulirus argus (Palinuridae). Journal of Applied Aquaculture 18(4): 33–43.
Creswell, R.L. 1984. Increased production of spiny lobsters (Panulirus argus) through post harvest impoundment. Proceedings of the Gulf and Caribbean Fisheries Institute. 43: 23–35.
Jeffs, A.G. and P. James. 2001. Sea-cage culture of spiny lobster Jasus edwardsii in New Zealand. Marine and Freshwater Research. 52: 1419–1424.
Jeffs, A.G. 2003. The Potential for Crayfish Aquaculture in Northland. NIWA Project No. ENT03101. Aquaculture Development Group Enterprise Northland. Auckland, New Zealand.
Jeffs, A. and M. Davis. 2003. An assessment of the aquaculture potential of the Caribbean spiny lobster, Panulirus argus. Proceedings of the Gulf and Caribbean Fisheries Institute. 54: 413–426.
Kittaka, J. 1997. Culture of larval spiny lobsters: a review of work done in northern Japan. Marine and Freshwater Research. 48(8): 923–930.
Lellis, W.A. 1991. Spiny lobster: A mariculture candidate for the Caribbean? World Aquaculture. 22: 60–63.
Lipcius, R.N. and D.B. Eggleston. 2000. Ecology and fishery biology of spiny lobsters. In: Spiny Lobsters:Fisheries and Culture. Eds B.F. Phillips and J. Kittaka. Pp. 1–41. Fishing News Books. Oxford.
Lozano-Alverez, E. 1996. Ongrowing of juvenile spiny lobsters, Panulirus argus (Latreille, 1804) (Decapoda, Paliunridae), in portable sea enclosures. Crustaceana. 69: 958–972.
Marx, J.M. and W.F. Herrnkind. 1986. Species Profiles: life histories and environmental requirements of coastal fishes and invertebrates (south Florida) spiny lobster. U.S. Fish and Wildlife Service Biological Report 82(11.61). U.S. Army Corps of Engineers, TR EL-82-4. 21pp.
National Marine Fisheries Service. NMFS Annual Commercial Landings Statistics. https://www.st.nmfs.noaa.gov/commercial-fisheries/commercial-landings/annual-landings/index
Power, R., J.L. Murno, M. Diffenthal, and G. Lane. [In Press]. Preliminary investigations into the feasibility of small-scale commercial aquaculture of Panulirus argus, based on collection of pueruli from the wild. Proceedings of the Gulf Caribbean Fisheries Institute.
Sharp, W.C., W.A. Lellis, M.J. Butler, W.F. Herrnkind, J.H. Hunt, M. Pardee-Woodring, and T.R. Matthews. 2000. The use of coded microwire tags in mark-recapture studies of juvenile Caribbean spiny lobster, Panulirus argus. Journal of Crustacean Biology. 20: 510–521.
Shields, J.D. and D.C. Behringer Jr. 2004. A new pathogenic virus in the Caribbean spiny lobster Panulirus argus from the Florida Keys. Diseases of Aquatic Organisms. 59: 109–118.
Sjoken, R. 1999. The Formulation and Evaluation of an Artificial Feed for the Growout of Spiny Lobsters. MS Thesis, Graduate School of the Florida Institute of Technology. Melbourne, FL.
Ting, R.Y. 1973. Culture potential of spiny lobster. In: Proceedings of the 4th Annual Workshop, World Aquaculture Society. Pp 165–170. Baton Rouge, Louisiana.
Witham, R., R.M. Ingle, and E.A. Joyce, Jr. 1964. Notes on postlarvae of Panulirus argus. Quarterly Journal of the Florida Academy of Science. 27: 289–297.
Witham, R., R.M. Ingle, and E.A. Joyce, Jr. 1968. Physiological and ecological studies of Panulirus argus from the St. Lucie estuary. Fla. Board. Cons. Mar. Res. Lab. Tech Ser. No. 53. 31 pp.
Witham, R. 1973. Preliminary thermal studies on young Panulirus argus. Florida Scientist. 36: 154–158.
Yeung, C. and M.F. McGowan. 1991. Difference in inshore-offshore and vertical distribution of phyllosoma larvae of Panulirus, Scyllarus, and Scyllarides in the Florida Keys in May-June 1989. Bulletin of Marine Science. 49: 699–714.