The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Mole crickets are the most important insect pests of turf and pastures in Florida, but their damage is not restricted to grasses. Their feeding and tunneling also destroy seedlings of vegetables, ornamentals, and tobacco. The insecticides commonly applied to control mole crickets in lawns, golf courses, and seed beds are expensive and not always effective. In pastures there is no control that is economically feasible; yet without control, stands of pasture grasses are frequently so reduced replanting is necessary.
In 1985, a nematode parasite of mole crickets was brought from Uruguay to Florida. That nematode, which was described as a new species, Steinernema scapterisci (Nguyen and Smart 1990), appears to be a major factor in limiting populations of mole crickets in South America.

Credit: K. Nguyen, University of Florida
Description
Male, First-Generation
A first-generation male is much smaller than a first-generation female, but anatomically the two are similar anteriorly. The body is usually plump and the head is rounded continuous with the body. Lips not seen but six labial papillae are prominent, and four cephalic papillae are well developed, much larger than cephalic papillae. Esophagus steinernematoid, procorpus cylindrical, metacorpus slightly swollen, isthmus distinct, basal bulb swollen with small valve. Nerve ring located in isthmus region of esophagus but position variable. Excretory pore located anterior to mid-esophagus. Excretory duct not forming elliptically shaped structure present in females. Posterior part of body curved ventrally. Body assumes a spiral shape when killed by minimal heat. Gonad single, testis reflexed. Spicules averaging 83 micrometers long, dark brown in color, paired, uniformly curved, with head large and somewhat angular. Angle formed by shaft and blade of spicules averages 110 degrees (range 100–120). Shaft of spicules long when compared to those of other species of the genus and appears to be encased in a sheath; blade tapers smoothly to end with posterior portion thinner than that for other species of Steinernema. A small aperture can be seen on ventral side close to tip of the blade. Each spicule has two internal ribs with variable termination point proximally. Gubernaculum boat-shaped, with anterior part thin, long and ventrally curved. Spicules glide along gubernaculum in two grooves separated by a needle-shaped cuneus. Cloaca on a raised area bearing an anterior flap, seen easily when the spicules are protracted or retracted. Eleven or 12 pairs and one single genital papillae observed. One special pair located at the edge of cloaca. Tail with a mucron. No phasmid observed.
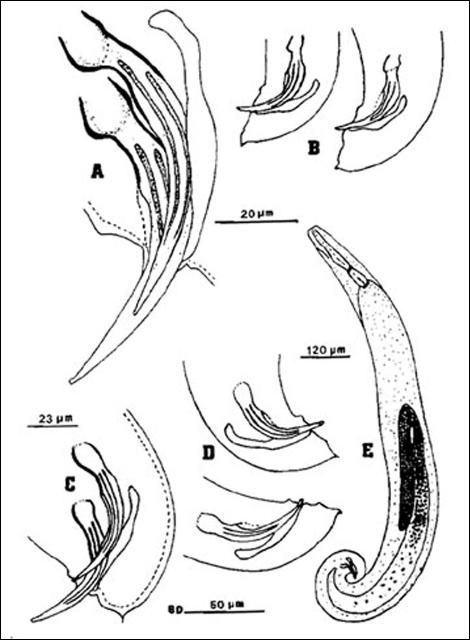
Credit: K. Nguyen, University of Florida
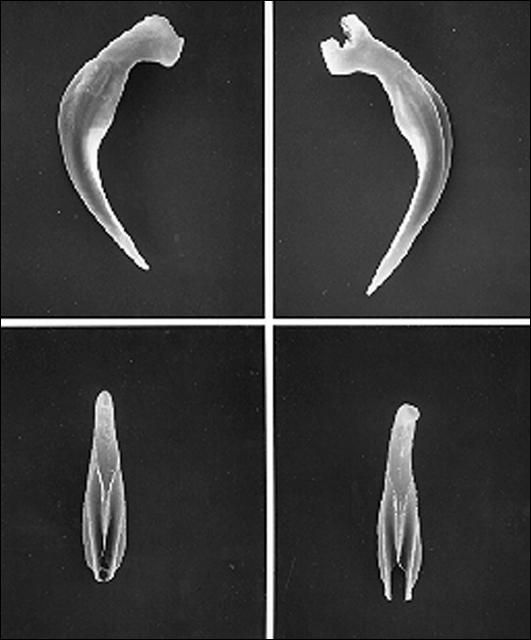
Credit: K. Nguyen, University of Florida
Male, Second Generation
A second generation male is similar morphologically to that of the first generation except that it is about two-thirds as long and one-half as wide and the spicules have an elongate head.
Female, First Generation
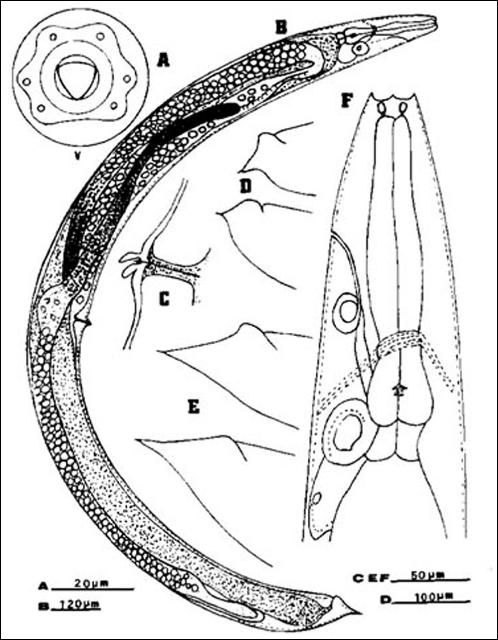
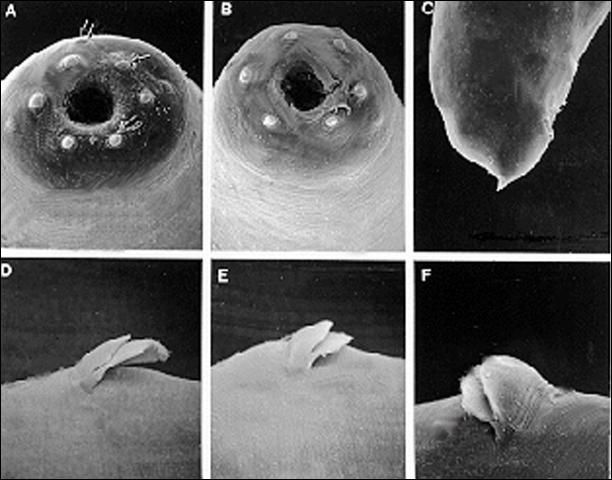
Anteriorly, the female is similar to the male but much larger. Lateral fields and phasmids not observed. Six labial papillae and four cephalic papillae present. Stoma with prominent cheilorhabdions unusually thickened, appearing as a circular or hexagonal ring in face view. Esophagus typical of family. Esophago-intestinal valve large. Excretory pore located anteriorly to mid-metacorpus.
Excretory duct unusually prominent forming an elliptically shaped structure seemingly with a hole at the center. Gonads didelphic, amphidelphic, reflexed. Vulva appears as a transverse slit with a prominent double-flapped epiptygma. Tail length shorter than anal body width, with a mucron at end.
Credit: K. Nguyen, University of Florida
Credit: K. Nguyen, University of Florida
Females, Second Generation
Second-generation female similar morphologically to that of the first generation with the following exceptions: about one-half as long and two-thirds as wide, valve in basal bulb of esophagus more prominent, elliptically shaped structure less prominent, tail, which tapers to a point bearing a mucron, longer than body width at anus.
Infective Juveniles (Third Stage)
The infective stage, when newly formed, is always enclosed in the cuticle of the second-stage juvenile as a sheath. However, the sheath is lost rather easily, even in storage, and thus may not always be present. Body thin, head with a labial raising disc; lip region not offset, oral aperture not observed; six labial, four cephalic papillae and an elevated oral disc prominent. Esophagus degenerate and thus not seen clearly, but its basal bulb is elongate and has a valve. Lateral field with six incisures. Tail tapers gradually dorsally but abruptly ventrally.
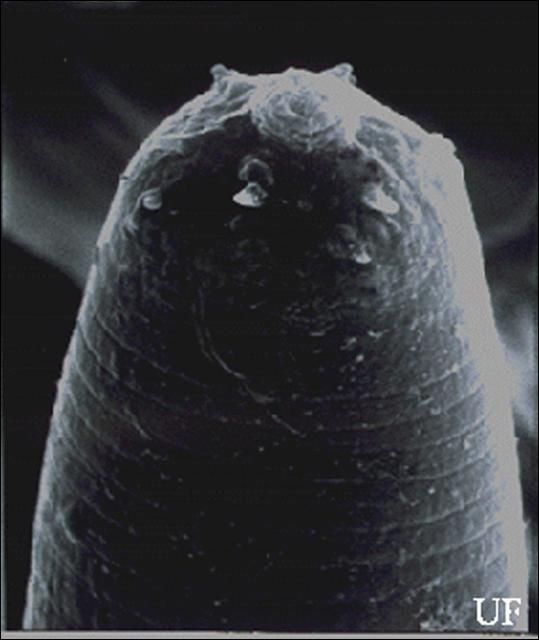
Credit: K. Nguyen, University of Florida
Biology and Life Cycle
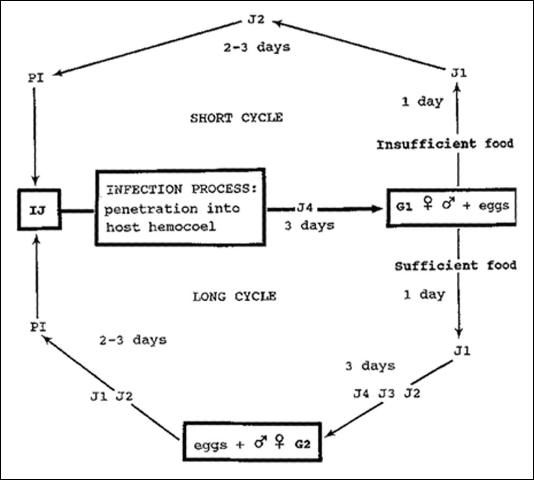
The life cycle of Steinernema scapterisci (Nguyen & Smart) consists of an egg stage, four juvenile stages, and an adult stage (male and female). The cycle from IJ (third stage infective juveniles) to IJ may proceed by one of two routes. If the nutrient supply is sufficient and the population is not overcrowded, the IJ develop to adult males and females of the first generation. Most eggs from these adult females hatch and the juveniles develop through each life stage to become adult males and females of the second generation. Eggs produced by these females develop to IJ. This cycle takes eight to 10 days (long cycle) at 24°C (75.2°F). If the nutrient supply is insufficient or if overcrowded, the IJ develop to adult males and females of the first generation, and eggs produced by the females develop directly to IJ. This cycle takes six to seven days (short cycle). The nematode is less tolerant of lower temperatures and more tolerant of higher temperatures than are other species of the genus. The sex ratio is influenced by temperature. At 15 (59) and 24°C (75.2°F), females constituted 54 percent and 60 percent of the population, respectively, but at 30°C (86°F) females constituted 47 percent of the population.
Distribution and Hosts
Steinernema scapterisci was found originally in Argentina, Brazil and Uruguay and was found recently in the United States. It was reported to infect and kill insects in the order Orthoptera: mole crickets of the genera Scapteriscus and Neocurtilla; house cricket, Acheta domesticus; field crickets, Gryllus spp.; eastern lubber grasshopper, Romalea guttata. Insects in the orders Coleoptera, Lepidoptera, Hymenoptera are poor hosts or non-hosts.

Credit: K. Nguyen, University of Florida
Release and Dispersal
The research to use entomopathogenic nematodes to control mole crickets began in 1983 by conducting a survey in attempts to find mole crickets infected with steinernematid or heterorhabditid nematodes. About two thousand mole crickets from Alachua County, Florida, and a few from other counties in the state, were collected and held until they died. Three to five days after they died the cadavers were dissected and examined for steinernematid or heterorhabditid nematodes. No heterorhabditids were found and only four were infected with steinernematids. One of those was infected with Steinernema glaseri, and three were infected with Steinernema carpocapsae. Progeny of these nematodes were used in laboratory experiments to infect other mole crickets. Neither of the species infected mole crickets well or reproduced well in those that became infected.
Steinernema scapterisci was not found in Florida before the release in 1985, when this nematode was applied to small plots in Alachua County, Florida. Of the mole crickets collected in pitfall traps during a 24-hour collection period immediately after application, a maximum of 40 percent were infected with Steinernema scapterisci. These plots were sampled monthly for five years and about 10 percent of the mole crickets trapped were infected. That 10 percent, coupled with the higher initial infection, was sufficient to reduce populations of nymphal mole crickets collected in pitfall traps by about 85 percent after one year and 95 percent or more thereafter. The nematode persisted in the plots for at least the duration of our five-year sampling period. The effectiveness of the nematode is due in part to the fact that the mole crickets have only one generation per year in northern Florida and the nematode, theoretically, could produce a new generation every 10 days for approximately eight months of the year.
Three years after the nematode was released, a few mole crickets collected on a farm 10 miles from the nearest release site were found infected with the nematode. By the fifth year, 25 to 65 percent of the mole crickets collected at this farm were infected (Smart et al. 1990).
Since 1985 Steinernema scapterisci has been detected in mole crickets collected from several other sites in Alachua County, undoubtedly spread from our original release sites by infected mole crickets. By November 1998, about 10 to 30 percent of mole crickets collected in Alachua County, Florida were infected with Steinernema scapterisci.
In 1990 and 1991, the nematode was released in pastures in six different counties and on nearly 30 golf courses in Florida. It has become established and has reduced populations of mole crickets in all of the pastures.
Bionomics and Host Parasite Relationships
The nematode can survive and still be infective in the absence of hosts for at least 10 weeks in moist soil (Nguyen and Smart 1990a). When infective juveniles (IJ) of Steinernema scapterisci were released on the soil surface in the field and in the laboratory, they moved downward through the soil at least 10 cm in five days, infected and killed mole crickets (Nguyen and Smart 1990b). When IJ were placed at the center of a 16-cm soil column, they moved in both directions with three times more moving downward than upward. In the field, nematode-infected mole crickets in the early stage of infection spread the nematode during flight (Smart et al. 1991). Consequently, the nematode can be dispersed from inoculative field releases on golf courses and pastures (Parkman et al. 1993a, 1993b). The IJ enter the host through the mouth or spiracles (Nguyen and Smart 1992).
Bacterial Associates
Bacteria from Steinernema scapterisci cultured in vivo, in vitro and collected from fields were isolated and identified (Anguillera et al. 1993a). Many bacteria were associated with this nematode: Ochrobactrum anthropi, Paracoccus denitrificans, Xanthomonas maltophilia, Xenorhabdus bovienii, Xenorhabdus nematophilus, and Xenorhabdus spp. These bacteria and three others, Pseudomonas fluorescens, Pseudomonas aureofacians, and Escherichia coli were used for in vitro culture of this nematode. The nematode developed normally and produced IJ with all studied bacteria (Anguillera et al. 1993b). This shows that the nematode can feed on different bacteria and survive very well under field conditions.
Biocontrol Capability
Steinernema scapterisci was successfully introduced by inoculative applications (Parkman et al. 1993, 1994) in Florida. Since 1993, this nematode has been commercialized in Florida to control mole crickets in golf courses and pastures.
Selected References
Anguillera MM, Smart GC Jr. 1992. Bacterial symbionts of Steinernema scapterisci. Journal of Invertebrate Pathology 62: 68-72.
Anguillera MM, Smart GC Jr. 1992. Development, reproduction, and pathogenicity of Steinernema scapterisci in monoxenic culture with different species of bacteria. Journal of Invertebrate Pathology 62: 289-294.
McSorley R. (July 1997). Soil-inhabiting nematodes. UF/IFAS Featured Creatures. EENY-12. http://entomology.ifas.ufl.edu/creatures/nematode/soil_nematode.htm (August 1999).
Nguyen KB. (May 1999). Mole cricket control by entomopathogenic nematodes. UF/IFAS. (no longer available online)
Nguyen KB. (February 1999). Taxonomy of entomopathogenic nematodes. UF/IFAS. (no longer available online).
Nguyen KB. (February 1998). Symbiotic bacteria of entomopathogenic nematodes. UF/IFAS. (no longer available online).
Nguyen KB, Smart GC Jr. 1992a. Life cycle of Steinernema scapterisci Nguyen and Smart, 1990. Journal of Nematology 24: 160-169.
Nguyen KB, Smart GC Jr. 1992b. Addendum to the morphology of Steinernema scapterisci. Journal of Nematology 24: 478-481.
Nguyen KB, Smart GC Jr. 1991a. Pathogenicity of Steinernema scapterisci to selected invertebrates. Journal of Nematology 23: 7-11.
Nguyen KB, Smart GC Jr. 1991b. Mode of entry and sites of development of Steinernema scapterisci. Journal of Nematology 23: 267-268.
Nguyen KB, Smart GC Jr. 1990a. Preliminary studies on survival of Steinernema scapterisci in soil. Soil and Crop Science Society of Florida, Proceedings 49: 230-233.
Nguyen KB, Smart GC Jr. 1990b. Vertical dispersal of Steinernema scapterisci. Journal of Nematology 22: 574-578.
Nguyen KB, Smart GC Jr. 1990. Steinernema scapterisci n. sp. (Steinernematidae: Nematoda). Journal of Nematology 22: 187-199.
Parkman JP, Frank JH, Nguyen KB, Smart GC Jr. 1994. Inoculative release of Steinernema scapterisci (Rhabditida: Steinernematidae) to suppress pest mole crickets (Orthoptera: Gryllotalpidae) of golf courses. Environmental Entomology 23: 1331-1337.
Parkman JP, Frank JH, Nguyen KB, Smart GC Jr. 1993. Dispersal of Steinernema scapterisci (Rhabditida: Steinernematidae) after inoculative applications for mole cricket (Orthoptera: Gryllotalpidae) control in pastures. Biological Control 3: 226-232.
Smart GC Jr, Nguyen KB. 1995. Biological control of Orthoptera pest insects. United State Patent, patent number 5,466,448, date of patent: November, 14 1995.
Smart GC Jr, Nguyen KB, Parkman JP, Frank JH. 1990. Biological control of mole crickets in the genus Scapteriscus with the nematode Steinernema scapterisci Nguyen and Smart, 1990. Rencontres Caraibes en lutte biologique, Guadeloupe, 5-7 November 1990. Ed. INRA, Paris 1991.