Introduction
The Japanese beetle, Popillia japonica Newman, is a widespread and destructive pest of turf, landscape, and ornamental plants in the United States. It is also a pest of several fruit, garden, and field crops and has a total host range of more than 300 plant species. Adult Japanese beetles feed on foliage, flowers, and fruits. Leaves are typically skeletonized or left with only a tough network of veins. The larvae, commonly known as white grubs, primarily feed on roots of grasses often destroying turf in lawns, parks, and golf courses. Currently the Japanese beetle is the most widespread pest of turfgrass and costs the turf and ornamental industry approximately $450 million each year in management alone (Potter and Held 2002).

Credit: Ronald S. Kelley, Vermont Department of Forests, Parks and Recreation, www.forestryimages.org
Distribution
Outside of its native Japan, Popillia japonica is found in China, Russia, Portugal, Canada and the United States (CABI 2009). Since the first detection in the United States in a nursery near Riverton, New Jersey, in 1916, it has spread to many states east of the Mississippi River (except Florida), as well as parts of Wisconsin, Minnesota, Iowa, Missouri, Nebraska, Kansas, Arkansas, and Oklahoma. In more recent studies, it has also been found in Texas, South Dakota, Washington, North Dakota, as well as a few spots in California, Oregon, and Nevada. However, the outbreaks in California, Oregon, and Nevada have reportedly been eradicated with chemigation (CABI 2008). Despite regulatory efforts, by 2002 it had become established in at least 30 states (status map) (More detailed status map). Of the states in the southern region, climatological studies predict that it will establish in all states bordering the Gulf of Mexico, (Johnson and Lyon 1991) although the beetle still remains unable to establish in Florida.
Favorable climate, availability of a wide variety of host plants, and lack of important natural enemies have influenced the spread of Japanese beetle in the United States (Fleming 1972). The expanding area of turfgrass has also provided excellent breeding ground for the beetles whose grubs continue to be the most damaging pest of turf in the northeastern US (Cranshaw 2004).

Credit: M.G. Klein, USDA, www.forestryimages.org
Description
The following description of Popillia japonica biology is based on the detailed account by Fleming (1972).
Adult
The adult is an attractive and broadly oval beetle, 8 to 11 mm long (1/3 to 1/2 inch) and 5 to 7 mm (~1/4 inch) wide with females normally being larger than males. It is generally metallic green, with bronze or coppery-brown wing covers that do not completely cover the abdomen. The five patches of white hairs on each side of the abdomen and one pair on the last abdominal segment distinguish Popillia japonica from all other similar looking beetles.

Credit: David Cappaert, Michigan State University, www.forestryimages.org
Egg
Newly deposited eggs may be spherical, ellipsoidal, or slightly cylindrical and usually have a diameter of about 1.5 mm. They may be translucent to creamy white with small hexagonal areas on the surface. During embryo development, the egg enlarges to double its initial size and becomes almost spherical.

Credit: David Cappaert, Michigan State University, www.forestryimages.org
Larva
Translucent and creamy white, the grub is covered with scattered long brown hairs interspersed with short, blunt spines. The head is yellowish-brown with strong dark-colored mandibles and the body consists of three thoracic and ten abdominal segments. Each thoracic segment bears a pair of segmented legs. Accumulation of fecal matter in the hindgut may give a grayish to dark appearance to the posterior end. As typical of a scarab larva, the grub is C-shaped when at rest.

Credit: John A. Weidhass, Virginia Tech, www.forestryimages.org
Pupa
Pupation takes place within an earthen cell formed by the last larval instar; the pupa is about 14 mm (1/2 inch) long and 7 mm (1/4 inch) wide. Its color ranges from pale cream to metallic green depending upon the age.

Credit: APHIS-USDA
Life Cycle
In most parts of its range, the Japanese beetle completes its life cycle in one year, but some populations in cooler climates may complete their development in two years (Vittum 1986). The appearance of adults, the timing of oviposition and subsequent development have been shown to vary with latitude and altitude and also from year to year (Fleming 1972). Adults emerge in mid-May in the warmer climates of Georgia and North Carolina. More northern populations in Massachusetts, New York, Vermont, and New Hampshire have adult emergence from late June to early July.
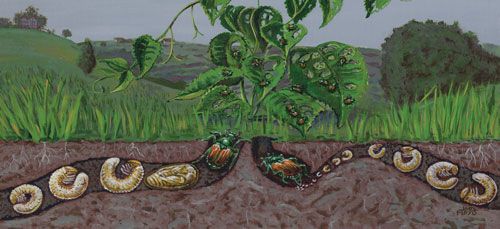
Credit: Joel Floyd USDA APHIS PPG
Males emerge a few days earlier than females, but eventually the population maintains a sex ratio of 1:1 (Fleming 1972; Régnière et al. 1981). Mating begins soon after emergence as virgin females release powerful sex pheromones that immediately attract large number of males. In an attempt to mate, the attracted males form a congregation around the unmated female, forming clusters referred to as beetle "balls," but mating rarely occurs under such intense competition (Ladd 1970).
Selection of a site for oviposition is influenced by proximity to host plant, nature of ground cover, and the soil condition. In suburban areas where turf is abundant, most beetles feeding on trees, shrubs, and vines deposit their eggs in the nearby grass (Fleming 1972). Although Popillia japonica generally lays most of its eggs on pastures, lawns and golf courses, eggs may also be deposited in agricultural fields. During dry summers when pastures are hard and dry, beetles are known to seek cultivated and fallow fields with loose and moist soil.
The ovipositing female burrows into the soil at a depth of 2 to 4 inches and deposits one to three eggs (singly). It will emerge the next day, or sometimes after three or four days, and continue to feed and remate. It may enter the soil more than sixteen times during its adult life and deposit a total of 40 to 60 eggs (Fleming 1972).
Eggs hatch in 10 to 14 days. The first instar feeds on nearby rootlets and organic matter for two to three weeks and molts for the first time. The second instar continues to feed for another three to four weeks and molts to a third instar. The majority of grubs reach the third instar by the fall when soil temperature gradually decreases. The activity of the grub ceases around 10°C (50°F) and most larvae overwinter as third instar at a depth of 5 to 15 cm (2 to 6 inches). With the beginning of spring, the grubs return to the plant roots to resume feeding for four to six weeks until they are ready to pupate. Pupation usually occurs near the soil surface and takes one to three weeks. Adults emerge from mid-May in warmer areas and June-July in cooler climates.
The life of adult beetles is relatively short under high temperatures and long under low temperatures (Fleming 1972). Studies with Japanese beetles under captivity have shown variations as wide as 9 to 74 days in males and 17 to 105 days in females; the generally accepted range is 30 to 45 days (Fleming 1972).
Hosts
More than 300 species of plants are known to be host to Japanese beetle. The following are some of the better-known primary and secondary hosts (CABI 2004).
Primary Hosts
Acer (maples), Asparagus officinalis (asparagus), Glycine max (soybean), Malus(ornamental species apple), Prunus (stone fruit including plums, peaches etc), Rheum hybridum(rhubarb), Rosa (roses), Rubus (blackberry, raspberry), Tilia (limes), Ulmus (elms), Vitis (grapes), Zea mays (corn).

Credit: USDA ARS, www.forestryimages.org

Credit: Clemson University, USDA Slide Series, www.forestryimages.org

Credit: Anne_Sophie Roy, European and Mediterranean Plant Protection Organization, www.forestryimages.org

Credit: Ariane McCorquodale, UF/IFAS

Credit: Clemson University, USDA Slide Series, www.forestryimages.org

Credit: Anne_Sophie Roy, European and Mediterranean Plant Protection Organization, www.forestryimages.org
Secondary Hosts
Aesculus (buckeyes), Althaea (hollyhocks), Betula (birches), Castanea (chestnuts), Hibiscus (rosemallows), Juglans nigra (American walnut), Platanus (planes), Populus (poplars), Salix (willow), Sassafras albidum (common sassafras), Sorbus americana (American mountain ash), turf grasses.
Wild Hosts
Lagerstroemia indica (crepe myrtle), Polygonum (knotweed/smartweed)
Damage
Both adults and larvae cause plant damage, but the host and nature of damage are usually different. Adults cause damage on foliage and flowers of a wide range of hosts and are most active on warm sunny days. The feeding on the upper leaf surface usually results in skeletonization. The grubs, which primarily feed on roots of grasses, cause considerable damage to pasture, lawn and golf courses. Feeding damage on roots reduces the ability of grass to take up enough water to withstand stresses of hot and dry weather and results in dead patches.

Credit: Ronald S. Kelley, Vermont Department of Forests, Parks and Recreation, http://www.invasive.org
Management
The aboveground feeding of adult Japanese beetle on multiple hosts, compared to the root feeding of grubs primarily on turf calls for different management strategy. In order to manage the Japanese beetle population, control efforts need to address both adult and larval population through an approach that integrates the following methods.
Physical Removal and Exclusion
In a small area, beetles can be physically removed from the plants on cool mornings when they are less active. They can also be collected in a bucket of soapy water by shaking the host plant (Ladd 1976). High value plants may be protected with nets during peak beetle activity.
Attractants and Trapping
Commercially available Japanese beetle traps are useful in reducing small, recently established, or isolated populations. However, their correct placement is important, as lures and traps placed adjacent to host plants attract more beetles and result in heavier damage (Gordon and Potter 1985). Even though these devices are most useful for monitoring populations and detecting new infestations, their deployment for mass trapping to suppress established populations is considered rather ineffective (Potter and Held 2002).
Cultural Control
During dry summers, female beetles seek irrigated and low-lying areas for oviposition since soil moisture is essential for egg survival and larval development. Withholding of irrigation during peak beetle flight activity may reduce grub population in turf (Potter et al. 1996). Powered rotovation of soil to a depth of at least 10 cm during drier conditions around fall has proven to minimize survival of larvae, along with the removal of host plants in smaller infestations (EPPO 2016).
Chemical Control
Since Japanese beetle is not yet reported as a pest problem in Florida, chemical recommendations are not currently available in the UF/IFAS Insect Management Guide. The USDA has provided on their website a very easy to follow and detailed handbook of ways to help curb and manage a Japanese beetle population through the use of IPM techniques and synergistic management options (USDA Japanese beetle handbook) Individuals in other states should also contact their county Cooperative Extension Service office for local recommendations.
Biological Control
Two species of tiphiid wasps, Tiphia vernalis Rohwer and Tiphia popilliavora Rohwer have proven successful biocontrol agents against Japanese beetle grubs (Fleming 1976). Tiphia vernalis attacks overwintering grubs, whereas Tiphia popilliavora attacks young grubs in late summer. A tachinid fly, Istocheta aldrichi (Mesnil), parasitizes adult Japanese beetles.
Ants and ground beetles feed on eggs and young larvae; moles, skunks, and raccoons also prey on the grubs although their foraging activity may often be destructive to turf (Potter 1998).
An entomopathogenic nematode, Steinernema kushidai (Mamiya), has been observed to cause mortality rates comparable to an organophosphate insecticide, diazinon (Koppenhöfer et al. 2000). It is specific to scarab larvae, and the effect of inundative releases have lasted in the field for one to two years. Its use in combination with other chemical products is known to produce a synergistic effect. Two other nematodes known to be most effective against Japanese beetle grubs are Steinernema glaseri (Steiner) and Heterorhabditis bacteriophora (Poinar). Proper application knowledge of entomopathogenic nematodes is required for maximum effectiveness, as improper application can result in greatly reduced efficacy because of nematode climate sensitivity.
Dusts containing spores of Bacillus popilliae (Dutky), the causal agent of milky disease have been used in the past with satisfactory results but isolate of Bacillus thuringiensis, designated as serovar japonensis strain Buibui (Btj), has subsequently been found to be more effective (Potter and Held 2002).
Selected References
CAB International. (2009). Crop Protection Compendium. https://www.cabi.org/cpc/ (04 February 2022).
Cranshaw W. 2004. Garden Insects of North America: The Ultimate Guide to Backyard Bugs. Princeton, NJ: Princeton University Press.
EPPO. (2004). Paris, France: European and Mediterranean Plant Protection Organization. PQR database (version 4.3). http://www.eppo.org/QUARANTINE/QP_insects.htm
EPPO. 2016. "Popillia japonica: Procedures for official control." European and Mediterranean Plant Protection Organization Bulletin 46: 543–556.
Fleming WE. 1972. Biology of the Japanese beetle. Washington, DC: USDA Technical Bulletin 1449.
Fleming WE. 1976. Integrating control of the Japanese beetles a historical review. Washington, DC: USDA Technical Bulletin 1545.
Gordon FC, Potter DA. 1985. "Efficiency of Japanese beetle (Coleoptera: Scarabaeidae) traps in reducing defoliation of plants in the urban landscape and effect on larval density in turf." Journal of Economic Entomology 78: 774–778.
Johnson WT, Lyon HH. 1991. Insects that Feed on Trees and Shrubs. Ithaca and London: Comstock Publishing Associates, Cornell University Press.
Koppenhöfer AM, Wilson M, Brown I, Kaya HK, Gaugler R. 2000. "Biological control agents for white grubs (Coleoptera: Scarabaeidae) in anticipation of the establishment of the Japanese beetle in California." Journal of Economic Entomology 93:71–87.
Krishik V. (2001). Japanese beetle management in Minnesota. University of Minnesota Extension Service. http://www.extension.umn.edu/ (09 January 2019).
Ladd TL Jr. 1970. "Sex attraction in the Japanese beetle." Journal of Economic Entomology 63: 905–908.
Ladd TL Jr. 1976. Controlling the Japanese beetle. USDA Home and Garden Bulletin 159.
NAPIS. (1998). National Agricultural Pest Information Service. Washington, DC, USA: USDA/APHIS. http://napis.ceris.purdue.edu (09 January 2019).
Potter DA. 1998. Destructive Insects: Biology, Diagnosis, and Control. Chelsea, MI: Ann Arbor Press. 344 pp.
Potter DA, Held DW. 2002. "Biology and management of Japanese." Annual Review of Entomology 47: 175–205.
Potter DA, Powell AJ, Spicer PG, Williams DW. 1996. "Cultural practices affect root-feeding white grubs (Coleoptera: Scarabaeidae) in turfgrass." Journal of Economic Entomology 89: 156–164.
Régnière J, Rabb RL, Stinner RE. 1981. "Popillia japonica: simulation of temperature-dependent development of the immatures, and prediction of adult emergence." Environmental Entomology 10: 290–296.
USDA-APHIS. (April 2004). Managing the Japanese Beetle. Federal Citizen Information Center. https://www.aphis.usda.gov/plant_health/plant_pest_info/jb/downloads/JBhandbook.pdf (09 January 2019).
USDA/ARS. (April 2010). Geraniums could help control devastating Japanese beetle. ScienceDaily. http://www.sciencedaily.com/releases/2010/03/100308132134.htm (09 January 2019).
Vittum PJ. 1986. "Biology of the Japanese beetle (Coleoptera: Scarabaeidae) in eastern Massachusetts." Journal of Economic Entomology 79: 387–391.