This article is part of a series entitled Soils and Fertilizers for Master Gardeners. The rest of the series can be found at https://edis.ifas.ufl.edu/topic_series_soils_and_fertilizers_for_master_gardeners. A glossary can also be found at https://edis.ifas.ufl.edu/MG457.
Introduction
Many turf and ornamental fertilizers marketed to homeowners contain phosphorus (P) because it is an important plant nutrient. However, the Florida Urban Turf Fertilizer Rule for home lawn fertilization limits the amount of P that can be applied to turfgrass (see the fact sheet on this topic published in 2007 by the Florida Department of Agriculture and Consumer Services http://www.flaes.org/pdf/Urban_turf_fact_sheet.pdf). The Urban Turf Rule was developed because misuse of fertilizers containing P can result in transport of P from the landscape, by rain or irrigation water, to nearby water bodies, which can potentially lead to pollution of the water body. Understanding how P behaves in the landscape can help maximize plant growth and minimize harmful losses of P. This document explains the P cycle and helps homeowners understand how P behaves in the environment. This will make maintenance of home landscapes or gardens easier and more sustainable.
The Phosphorus Cycle
Sources of Phosphorus
To begin, consider grass clippings left on the lawn surface after mowing. Grass clippings contain forms of P that are not available for uptake by growing plants. However, as they decay, soil microorganisms, like bacteria and fungi, change the unavailable forms of P into forms that plants can use. These forms of P can then be dissolved in the water held in the soil (soil solution) and can be taken up by the roots of the growing grass. When the grass is mowed again, this cycle repeats (Figure 1). This description is a very simple example of the P cycle. We will now go into more detail.
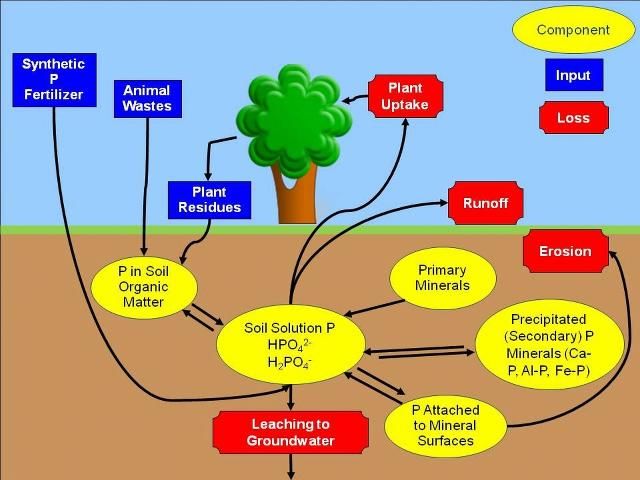
Credit: Amy L. Shober
Decaying plant materials are not the only source of P in the soil (Figure 1). When P recycling does not occur fast enough to supply enough available P to plants, fertilization may be needed for maximum plant performance. Thus, the fertilizer industry manufactures lawn and ornamental fertilizers for use by homeowners. Most of these materials are derived from phosphorus rich rocks that were deposited in ancient sea beds many years ago. This phosphorus rich material is mined from the earth and processed into more soluble P forms that plants can use. The ability of these synthetic phosphorus fertilizers to dissolve in soil water depends on the chemical form.
Organic soil amendments like compost, peat, or manure contain phosphorus in plant available (soluble P), slowly available (phosphorus minerals and P attached to mineral surfaces), and plant unavailable forms. These materials are typically derived from plant residues or animal wastes. Like the grass clippings in our earlier example, the forms of P in these materials that are not available to the plant must be converted to soluble P by soil microbes.
How Does Phosphorus Behave in the Soil?
Phosphorus must be dissolved in the soil solution in order to be taken up by plant roots. The dissolved forms of plant-available P in the soil solution are called orthophosphates (H2PO4- or HPO42-, depending on the soil pH). The amount of P dissolved in the soil solution at any particular time is usually very small.
Once plant roots remove P from the soil solution, it is replenished by the residual P in the soil. We previously discussed how soil microbes transform organic forms of P in plant residues or organic soil amendments into plant-available P. This process is called mineralization, and the end products are soluble orthophosphates. Once in the soil solution, the orthophosphate form of P can be taken up by plant roots. The soil solution can also be replenished from several pools of inorganic (mineral) P in the soil. Solid P minerals in the soil can dissolve in the soil solution when concentrations of soluble P diminish. This process, called dissolution can be compared with adding sugar to a glass of iced tea. When solid sugar is added to tea, it will dissolve in the liquid. Phosphorus can be attached to soil particles such as clay or specific minerals that contain iron or aluminum. Phosphorus can detach from these soil particles, thereby supplying P to the soil solution via a process called desorption. Finally, solid rocks can be a source of P as they break down into soil over a long period of time by a process called weathering.
Just as soil solution P can be replenished when the concentration of P becomes low, P can be removed from the soil solution if the amount of P in the soil solution gets too high. Consider the iced tea example again. If too much sugar is added to the tea, some of it will not dissolve and will remain in solid form at the bottom of the glass. Similarly, when concentrations of P in the soil solution are too high, some of the dissolved P will form solid P minerals by a process called precipitation. Depending on soil pH, precipitation can result in the formation of solid calcium phosphate minerals (high soil pH) or aluminum and iron phosphate minerals (low soil pH). Alternatively, P can be removed from the soil solution and attach to soil particles like clays or iron and aluminum-bearing minerals via a process called adsorption.
Phosphorus and Water Quality
Phosphorus can move from the soil to surface or ground water as the landscape drains following heavy rain or excessive irrigation. When the rainfall or irrigation rate is higher than the soils ability to absorb water, the result is runoff. Runoff can transport soluble P, P attached to eroding soil particles, grass clippings and/or any recently applied landscape fertilizers into lakes, ponds, streams, rivers and bays. In addition, when the concentration of P in the soil exceeds the natural soil P holding capacity, P can be carried downward, or leached, through the soil. Florida's sandy soils have notoriously low P-holding capacity. Once P is leached, it may eventually reach groundwater or be discharged by our springs.
Once transported into water bodies or groundwater, inorganic P can become a water pollutant. For example, P lost in runoff or leachate may contribute to eutrophication of Florida's surface water bodies. Eutrophication is the enrichment of water with nutrients that results in excessive aquatic plant (mostly algae) growth. With time, oxygen depletion of eutrophic waters can lead to fish kills and their accompanying foul smell. Eutrophic water bodies can often no longer be used for fishing, swimming, boating or other recreational activities.
Can I Test My Soil for Phosphorus?
Phosphorus is one of the elements for which soil testing is useful. When testing is performed by a reputable agricultural laboratory, the results indicate the relative availability of soil P. This will help you properly manage nutrients in your home landscape. Soil test results from the UF/IFAS Extension Soil Testing Lab (http://soilslab.ifas.ufl.edu) will include recommendations for P fertilizer rates for turfgrass and ornamental plants, if fertilization is indeed necessary. It is important that you follow P fertilizer recommendations to protect water quality. Contact your local UF/IFAS Extension office or see the EDIS publication Landscape and Vegetable Garden Test Information Sheet (https://edis.ifas.ufl.edu/SS187) for more information about soil testing and taking a soil sample.
How Can You Help Protect Water Quality
As a homeowner in Florida, you can help protect water quality by following best management practices (BMPs) when using ornamental or turf fertilizers. First and most importantly, fertilize only when your plants need nutrients (e.g., times of active growth) and follow UF/IFAS recommendations for rates and timings. For information on fertilization requirements for Florida lawns, see the EDIS publication Figuring Out Fertilizer for the Home Lawn (http://ufdc.ufl.edu/IR00003315/00001). The Urban Turf Fertilizer Rule is designed to reduce N and P losses and water pollution that can occur when fertilizers are not applied correctly. This new law will require fertilizer applicators (e.g., homeowners, landscape professionals) to follow specific guidelines for N and P application rates. For more information on this rule, see the EDIS publication Urban Turf Fertilizer Rule for Home Lawn Fertilization (https://edis.ifas.ufl.edu/EP353). Finally, if you spill fertilizer on the sidewalk or driveway, sweep it up and put it back in the bag, or apply it to target plants. Any fertilizer left on hard surfaces could easily be washed into a storm drain and discharged directly into a pond or stream. Similarly, sweep grass clippings back onto the lawn, because they contain P that also can be carried to the storm drain in runoff.
References
Brady, N. C., R. R. Weil. 2002. The Nature and Properties of Soil. 13th ed. Prentice Hall, Upper Saddle River, NJ.
J. L., J.D. Beaton, S. L. Tisdale, and W. L. Nelson. 2005. Soil Fertility and Fertilizers: An Introduction to Nutrient Management. 7th edition. Pearson Prentice Hall, Upper Saddle River, NJ.