Introduction
Organic matter is a complex material that represents the long-term decay products from plants and other organisms in the soil. When organic matter is allowed to build up in a soil, the soil color at the surface usually turns a darker color, often with a red or brown hue. Typically in Florida mineral soils, organic matter content is quite low, within the range of 1 to 5%. However, in some soils that remain flooded for most of the year, organic matter can build up with time and actually become the soil. Such is the case for the organic soils, or histosols, found in southern Florida. These organic soils comprise much of the Water Conservation Areas, Everglades National Park (ENP), Big Cypress Basin, and the Everglades Agricultural Area (EAA).
It is important to document organic matter accumulation in the Everglades to gauge the effectiveness of wetland creation and succession. For the EAA, the drained soils lose organic matter due to oxidation, so measurement of the organic matter content of these soils over the course of time indicates the oxidation potential and mineral incorporation from bedrock. Due to the wide diversity of soil types and methods of measuring soil organic matter, there is a need to devise a more universal method applicable to many types of histosols in south Florida.
The intent of this publication is:
- To describe a simple laboratory method for determining the organic matter content of the organic soils of southern Florida and demonstrate the importance of using this new procedure for improved accuracy and precision;
- To utilize this updated laboratory procedure for field sites across Everglades wetlands and the EAA; and
- To recommend this procedure be used by growers, state and federal agencies, and university and agency researchers dealing with the management of organic soils in southern Florida.
The target audiences for this publication are growers within the EAA; state and federal personnel dealing with organic soils in southern Florida; and researchers, Certified Crop Advisers, and other consultants.
A Short History of the Formation of Organic Soils in Southern Florida and the Effects of Land Use
Organic matter found in natural areas of the Everglades and the farm fields of the EAA is the result of plant residue buildup in soils that have been frequently flooded with geologic time. Drainage of the EAA has allowed for high crop productivity from these organic soils; however, oxidation of the organic matter has accelerated a process termed subsidence. In effect, microbial activity in drained soils, either in natural or active farming conditions, breaks down the organic matter and releases nutrients, byproducts, and greenhouse gases such as carbon dioxide. The result is a decrease in the depth of the organic topsoil with time, with up to several meters lost in some parts of the EAA. This process occurs in natural or farmed areas during the typical Florida dry season (October through May). The extent to which this process occurs is much higher for cultivated soils compared with most natural or undisturbed organic soils, such as wetlands. The subsidence rate in farming operations is a function of the depth to the water table and the time that the soil remains drained (aerated), which is much longer than similar soils located in wetlands.
Much of the southern Florida organic soils have developed over limestone deposits. Farming operations mix the soil profile, often including some of the underlying limestone, bringing the limestone closer to the surface (Figure 1). As limestone dissolves, the organic topsoil pH changes from acidic to basic. This pH change is brought about by the reaction of the carbonates in the limestone with acid-forming organic and inorganic compounds. This process is the same as that used by growers for many mineral soils throughout Florida, which is in effect liming the acidic soil to increase the soil pH to favor crop production. However in organic soils, there is really no need to lime the soil. In fact, liming is not recommended since the calcium associated with limestone can decrease the availability of nutrients such as phosphorus. Said another way, limestone that is brought up into the topsoil adversely affects the availability of phosphorus and other nutrients to crops growing on the land. Growers are then forced to use higher amounts of phosphorus fertilizers to obtain optimum nutritional responses and to satisfy the crop nutrient requirements. A decrease in the organic matter content of soil over time indicates an increase in the mineral content, which is predominantly calcium carbonate. Thus, there are relationships between the organic matter content, soil pH, and phosphorus cycling.

In wetlands, the process of subsidence is often considerably slower or even nonexistent due to the longer duration of flooding for most of the year. However, the presence of the underlying limestone on which the organic soil developed can still affect soil pH. Additionally, the restoration of these natural lands influences the rate of carbon sequestration or organic soil accumulation by transferring organic matter to the recalcitrant form of carbon, which scientists often express as organic carbon. In turn, organic carbon is related to the soil's organic matter content, usually by a constant that can change based upon the assumptions used to calculate that constant. The conversion of organic carbon values to soil organic matter content can be difficult because the carbon content of organic matter is not consistent and varies with vegetation type, degree of decomposition, age, and other factors, although 51% carbon in organic matter is a typical representation (Batti and Bauer 2002).
Using Laboratory Methods to Measure Organic Carbon
Soil organic matter has long been recognized to be important for optimal crop growth. Several chemical tests have been devised to measure soil organic matter, with the most useful proving to be accurate and precise. Unfortunately, these widely-accepted chemical tests are going out of favor because they utilize chromium, which is quite toxic if not disposed of properly. A more recent test involves the use of modern instrumentation, such as a Carbon-Nitrogen-Sulfur (CNS) Analyzer. While this laboratory instrument is complex, in reality, the theory is simple and involves measurement of gases evolved from heated soil samples. As organic matter is combusted at high temperature, the organic carbon is converted into carbon dioxide, which is then measured. Using this type of instrument, most soils can be successfully analyzed for organic carbon. Drawbacks to this method are the high costs of the instrument and its operation.
Another method also uses heat to slowly oxidize (burn) the organic matter out of the soil samples for 550°C for 4 hours. The Loss-on-Ignition (LOI) value for organic matter is then measured as the difference in the weight of the sample before and after oxidation. The assumptions around this much simpler and less costly measurement are critical to the reproducibility and repeatability of the LOI method.
Known Interferences for the CNS and LOI Methods
Limestone (calcium carbonate) already contains carbon in its chemical composition. This carbonate crystal can break down to some extent during either CNS or LOI analysis producing an overestimation for organic carbon, since the carbon from limestone is also included in the measurement.
Both methods can be rendered inaccurate and imprecise if selected mineral constituents are present. The CNS analyzer measures total carbon, which includes organic carbon and carbonates, but also requires the measurement of inorganic carbon which then has to be subtracted from the total carbon.
Gypsum, calcium sulfate crystals that also contain water molecules, is an example of a mineral that can contribute to error using LOI methods. In effect, heating the soil sample can drive off the crystalline water molecules, and cause an over-estimation of the organic carbon in the soil. So there are some potential drawbacks to both the LOI and CNS methods.
Results of Experiments using CNS and LOI Methods
Since CNS is currently the standard method for analyzing organic carbon and LOI is so much cheaper than CNS, a large study involving more than 3000 soil samples was conducted for the southern Florida wetlands (ENP, Water Conservation Areas, and Big Cypress Basin). Results of soils prepared using standard methods and then analyzed by both CNS and LOI were compared.
Total calcium concentration was also determined for all samples. Total calcium is much different than extractable calcium used in traditional soil testing for making fertilizer management decisions. The entire contents of the soil are destructively sampled to determine all of the calcium within the soil from all forms (exchangeable, non-exchangeable, within minerals, etc.). The total calcium in the soil might be used as a correction for carbonates since the bulk of calcium in organic soils is associated with limestone. Data values having units of g/kg can be converted to ppm by multiplying by 1000, or to % by dividing by 10.
Findings showed that:
- There was general agreement between LOI and CNS methods in estimating organic carbon.
- The LOI estimate could be improved greatly by taking into account the total calcium in the system whenever the LOI was less than 400 g kg-1, or when there was considerable mixing of calcium carbonate into soil.
- The CNS estimate was also influenced by the carbon contribution of carbon from limestone. By subtracting the carbon originating from limestone from the CNS estimate, a more accurate result for total organic carbon was calculated.
When researchers looked at the total calcium concentration, they found that calcium was a good indicator of where limestone was mixed with the soil. However, the relationship between Ca was different for CNS (Figure 2) and LOI measurements (Figure 3).
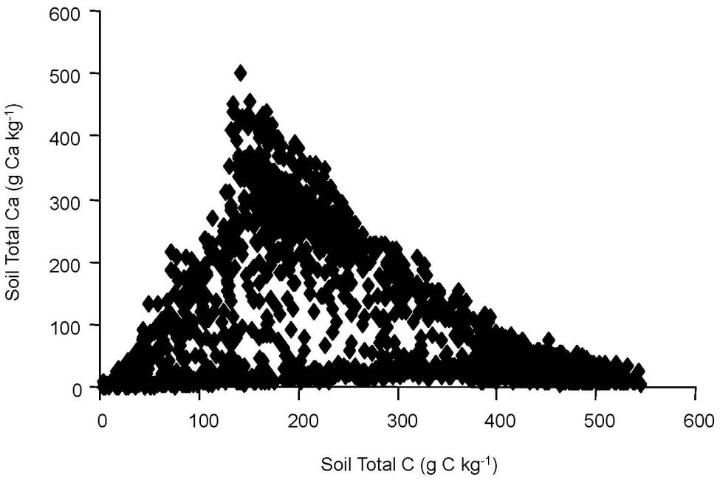
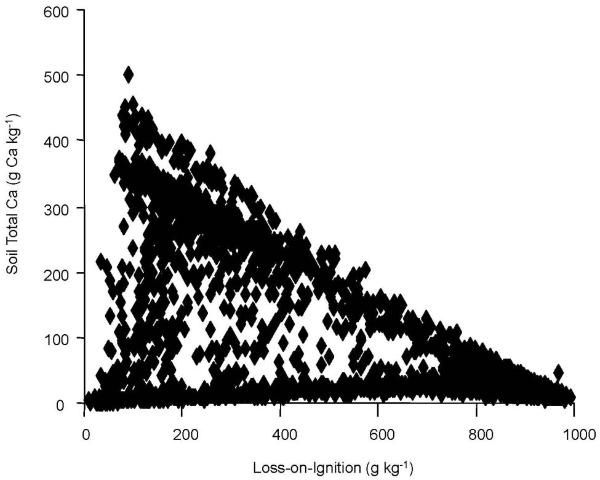
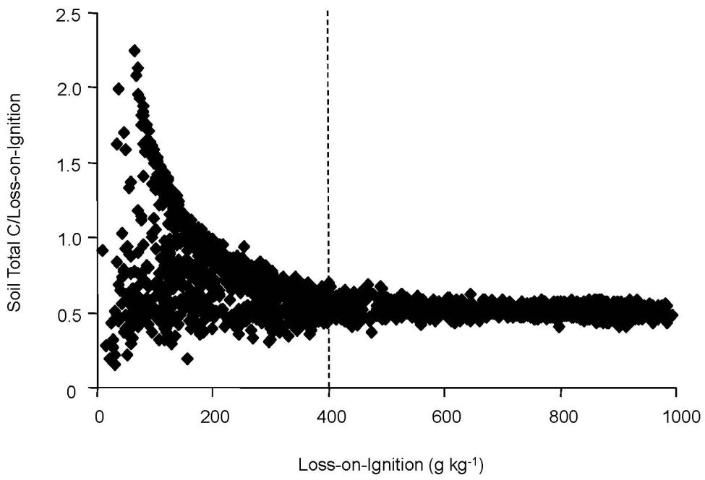
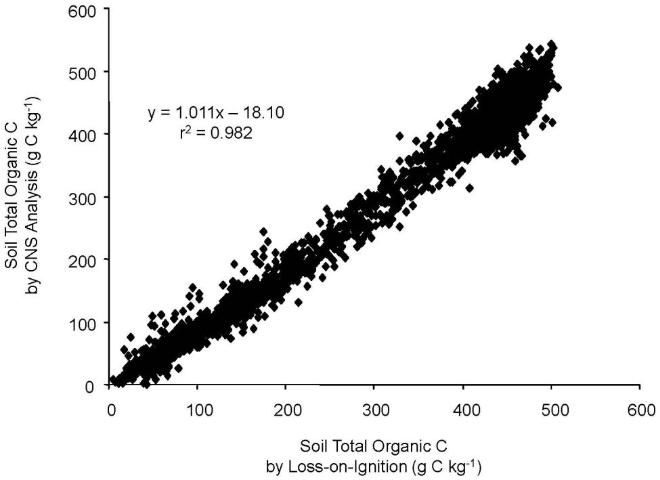
This difference between the two methods and total calcium also pointed out that total soil calcium could be used to adjust the results of both methods to improve accuracy. For example, researchers found that for LOI results less than 400 g kg-1 carbon, the presence of additional carbon from limestone is suggested by the wide range in ratios from 0.2 to 2.4 (Figure 4). This divergence, the researchers surmised, was due to carbon entering into the LOI measurement not from organic sources, but from limestone. After applying the adjustment for calcium originating from limestone, good agreement between the two methods was achieved (Figure 5).
Adjustments can be made easily to LOI measurements by taking into account the total soil calcium concentrations using formulas in Table 1. The first equation should be used when results from both LOI and total soil calcium are available. This equation works throughout the entire range of organic carbon tested in samples from southern Florida.
If total soil calcium is available and the LOI range is found to be in the 400 to 1,000 g kg-1 range, then the second equation should be used to adjust LOI to total carbon. However, the third equation can be used accurately if total soil calcium is not available.
Lastly, the fourth equation should be used to adjust LOI results when LOI is less than 400 g kg-1. In this case, total soil calcium is required since the low organic carbon and the high carbonate content are both contained in the LOI value. The contribution of the carbonates should be removed, as estimated by the total soil calcium.
Conclusions
Growers can use this improvement to organic matter measurement to keep lab testing costs low while getting a better, more quantitative estimate of organic carbon (organic matter) for decisions regarding pesticide applications and estimated contribution of nutrients released from the organic matter in their fields. Restoration efforts in the Everglades wetlands can be better documented with the lower cost, but now equally as useful, LOI test for organic carbon. Improvements to soil organic matter coupled with other measurements of biological health of the system can be documented with less work using the adjusted LOI calculations.
For More Information
Batti, J.S., and Bauer, I.E. 2002. "Comparing loss-on-ignition with dry combustion as a method for determining carbon content in upland and lowland forest ecosystems." Commun. Soil Sci. Plant Anal. 33:3419–3430.
Wright, A.L., Y. Wang, and K.R. Reddy. 2008. "A loss-on-ignition method to assess soil organic C in calcareous Everglades wetlands." Commun. Soil Sci. Plant Anal. 39:3074–3083.