Disease outbreaks caused by the oomycete fungal-like pathogen Phytophthora capsici (P. capsici) occurred sporadically in Florida prior to 1993. Since 1997, the disease has become a chronic problem for pepper and summer squash production in the southern and east central parts of the state. Phytophthora capsici can cause extensive losses, not only on pepper and summer squash, but also on eggplant and watermelon partly due to the rapidity of disease spread during favorable weather in Florida. The pathogen has caused severe epidemics in Central and South America, Europe, Asia, and many other states in the United States where susceptible vegetables are grown. The host range of P. capsici is wide and includes bell pepper, cacao, cantaloupe, chayote, cucumber, eggplant, honeydew melon, marigold, macadamia nut, papaya, pumpkin, some bean types, squash, tomato, and watermelon. Diseases caused by P. capsici are referred to as Phytophthora blight, Phytophthora crown and root rot, and Phytophthora fruit rot.
Symptoms
Phytophthora capsici causes seed rot and seedling blight in many solanaceous crops (pepper, eggplant, tomato) and cucurbits (cantaloupe, cucumber, summer squash, pumpkin, watermelon), similar to those seen with fungi, Pythium spp., and other Phytophthora spp. Rotting of seedlings prior to emergence (preemergence damping-off) and blighting of recently emerged seedlings (postemergence damping-off) can occur. The roots and plant base may be discolored and infected seedlings often topple over. White fungal-like (mycelia) growth may cover infected areas of blighted seedlings under moist conditions. On mature plants, P. capsici, as well as other Phytophthora spp., can also produce a wide variety of symptoms that vary by host. Although some bean types (broad, butter, lima, snap) can be susceptible to P. capsici, (Gevens et al. 2008), to date, there have been no reports of this occurring in Florida. Symptoms on bean include water-soaked foliage, stem and pod necrosis, and white mycelia or sporulation on the plant surfaces.
Pepper
Roots, stems, foliage, and fruit of mature pepper plants are susceptible. Although infection can occur at any height on stems, it is most common at the soil line and starts as a dark, water-soaked area. Stem lesions become dark brown to black and result in girdling and plant death (Figure 1). Infected roots are dark brown and mushy.

Leaf spots are small at first, irregular to round, and water-soaked. With age the spots enlarge, turn a light tan, and may crack. Infected areas may be bordered by white fungal-like growth during wet periods. Rapid blighting of new leaves and the entire emerging shoot may take place (Figure 2).

Pepper fruits are infected through the stem. Fruit rot appears as dark green, water-soaked areas that become covered with white-gray, cottony, fungal-like growth (Figure 3). Infected fruit dries, becomes shrunken, wrinkled, and brown, and remains attached to the stem.

Eggplant
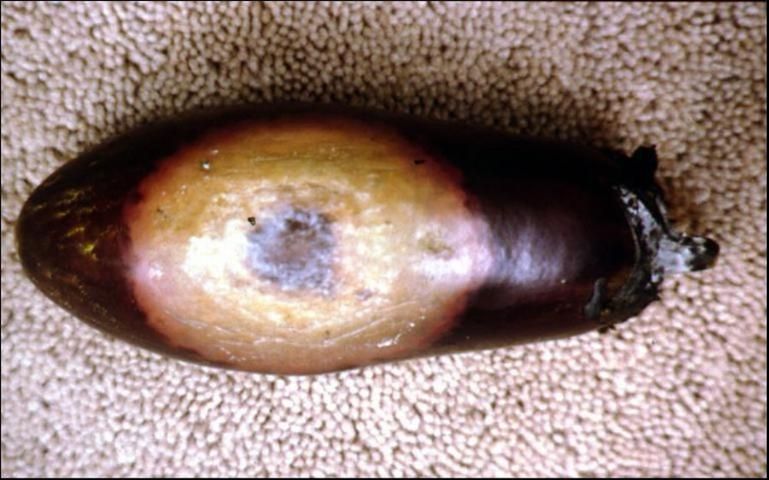
Although the entire plant may be susceptible, fruit rot is the primary symptom caused by P. capsici in eggplant. It begins as a round, dark brown area on any part of the fruit at any stage of maturity. The initial lesion is surrounded by a rapidly expanding light tan region (Figure 4). White to gray fungal-like growth may appear during wet, humid periods, starting on the oldest part of the fruit lesion. Phytophthora fruit rot in eggplant lacks the concentric patterns and dark fruiting structures present with Phomopsis rot, a fungus that also causes fruit rot on eggplant in Florida. Fruit rot in eggplant may also be caused by other Phytophthora spp. and Phomopsis sp.
Tomato
Phytophthora capsici can cause crown infections, leaf spot, and foliar blight in tomato. Diseased crowns are brown and soft and the plant may wilt and topple over (Figure 5). Another common symptom is fruit rot. Uninjured fruit of any age may be infected. Rot is most prevalent where fruit contacts the soil and begins as dark, water-soaked spots. The spot rapidly expands during warm weather and covers 50% or more of the fruit surface with a brown, water discoloration which may assume the appearance of concentric rings. At first, infected fruit remains smooth and firm even though the discoloration extends to its center. Over time and under humid conditions infected fruit may be covered with white fungal growth and rot entirely following invasion by secondary microorganisms. The symptoms of fruit rot in tomato caused by three other Phytophthora spp. (P. dreschlera and P. nicotianae, and P. parasitica) are essentially the same. Tomato fruit rot caused by P. infestans (late blight) is characterized by wrinkling and a definite, sunken margin.

Squash
Summer (yellow crookneck, zucchini) and winter (acorn, butternut) squash types are highly susceptible to Phytophthora foliar blight and fruit rot. Early foliar symptoms include rapidly expanding, irregular, water-soaked lesions in leaves. Dieback of shoot tips, wilting, shoot rot, and plant death quickly follow initial infection (Figure 6). Sunken, dark, water-soaked areas appear in infected fruit and are rapidly covered by white fungal growth in yellow summer squash and zucchini (Figures 7 and 8).



Watermelon
Watermelon foliage is less susceptible to P. capsici than that of summer squash. However, all stages of watermelon fruit are highly susceptible. Early symptoms of fruit rot include rapidly expanding, irregular, brown lesions which become round to oval. Concentric rings may occur within a lesion. The centers of rotted areas are covered with grayish fungal-like growth, while the outer margins of lesions appear brown and water-soaked (Figure 9). The entire fruit eventually decays. Initial symptoms of bacterial fruit blotch of watermelon are similar to those caused by P. capsici. However, after lesions expand, the two diseases can be easily separated because of the presence of extensive rind cracking, absence of fungal-like growth, and other symptoms associated with bacterial fruit blotch. Phytophthora rot is characterized by abundant fungal-like growth accompanied by little or no cracking.

Other Cucurbits
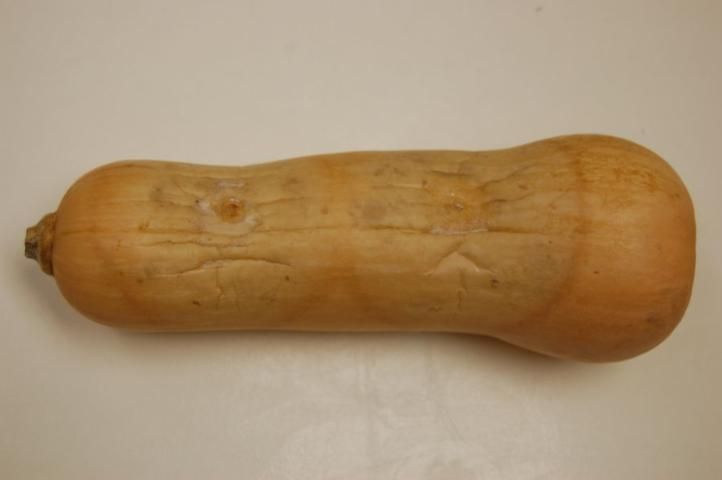
Phytophthora capsici causes rapid blighting and death of chayote and tropical pumpkin plants and a fruit rot similar to that observed in watermelon (Figure 10). Angular, water-soaked lesions as well as a rapid fruit rot, which is covered with white fungal-like growth, are produced in cucumber and butternut squash (Figure 11). Symptoms of Phytophthora blight in cantaloupe are limited to leaf lesions and tip dieback of vines.

Disease Cycle
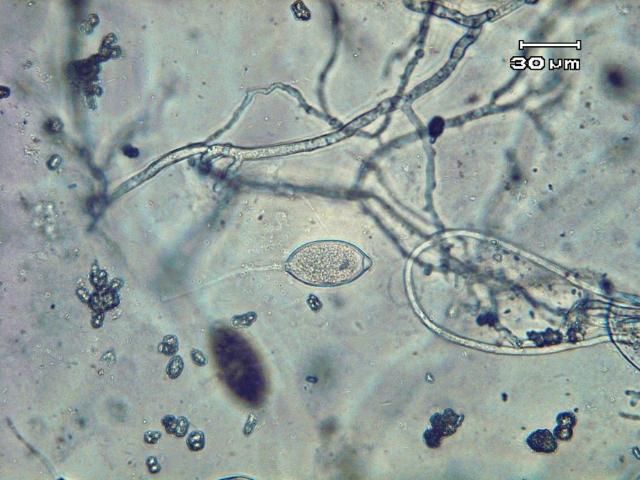
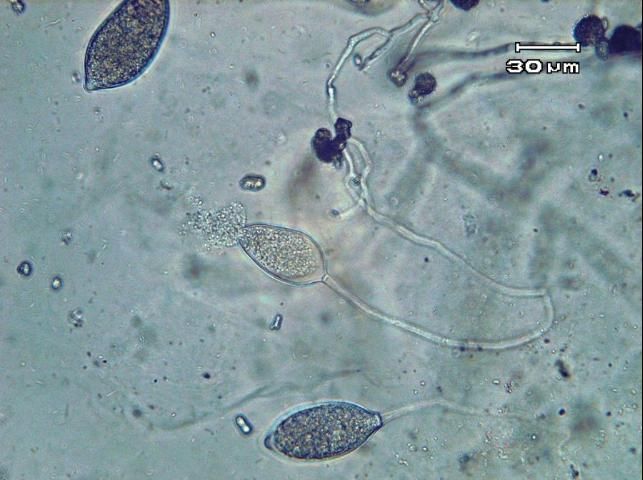
Phytophthora capsici may survive in and on seed and host plant debris in the soil by means of thick-walled spores (oospores). Certain weed species (Carolina geranium, American black nightshade, and common purslane) have been found to serve as alternative hosts for P. capsici in Florida (French-Monar et al. 2015). Surface water, in retention ponds or canals, may also be a potential inoculum source if water is used for field irrigation (Roberts et al. 2005). Both mating types of the pathogen necessary for oospore production have been identified in Florida production fields, often from the same field (Ploetz et al., 2005). The pathogen produces spores of another type called zoospores that are contained within sac-like structures known as sporangia (Figures 12 and 13). Zoospores are motile and swim to reach and invade host tissue. Plentiful surface moisture is required for this activity. The sporangia are spread by wind-blown rain through the air and are carried with water movement in soil. The pathogen is also moved as mycelia (microscopic, fungal-like strands) in infected transplants and through contaminated soil and equipment. Since water is integral to the dispersal and infection of P. capsici, maximum disease occurs during wet weather and in low, water-logged parts of fields. Excessive rainfall, such as that which occurs during El Niño years, coupled with standing water creates ideal conditions for epidemics caused by P. capsici. Growth of this pathogen can occur between 46°F–99°F (7°C–37°C), but temperatures between 80°F–90°F (27°C–32°C) are optimal for producing zoospores and the infection process. Under ideal conditions, the disease can progress very rapidly, and symptoms can occur 3–4 days after infection. Therefore, P. capsici can rapidly infect plants throughout the entire fields.
Management
Management practices in transplant production areas include the use of pathogen-free and fungicide-treated seed and sterile potting media. Flats, plug trays, benches, seeding equipment, and plant house structures should be disinfested using a sodium hypochlorite solution or other disinfestant. Steam sterilization of flats and plug trays may be useful. Transplant trays with infected plants should not be transported to field production sites. Workers should disinfest their hands after contact with infected plants before resuming their duties. Planting sites should be well drained and free of low-lying areas. The drainage area of the field should be kept free of weeds and volunteer crop plants, particularly those in solanaceous and cucurbitaceous groups. A preplant, broad-spectrum fumigant should be used for planting sites in the field. However, unfumigated soil areas in and near the field can serve as sources of recontamination. Rotating to non-susceptible crops may limit the amount of disease inoculum residing in the soil, debris, and weeds. Equipment should be decontaminated before moving between infested and noninfested fields. Once a field is infested, it will remain infested for many years and should be avoided if possible. Infected fruit should be culled to prevent spread in the packing house and during shipment. Culled fruit should be destroyed so as not to become another source of contamination for the farm area. It is best to bury culls in an area that will not be used for agriculture. Effective, labeled fungicides should be used preventively according to label instructions. It is essential that fungicides with different modes of action be rotated to prevent the buildup of fungicide resistance in P. capsici. Rotating or tank-mixing a systemic with a contact fungicide is a good fungicide resistance management tool.. Refer to EDIS publication Vegetable Production Handbook of Florida (https://edis.ifas.ufl.edu/cv292) for fungicides currently labeled for this disease on the crop of interest. Resistance to this disease has not been identified in cultivars currently grown in Florida. Foliar fungicides will minimize infection that occurs above the soil surface, and fungicide that are labeled for soil applications, such as through the drip irrigation system, can help manage the crown and root rot phase on labeled crops.
References
Gevens, A. J. Donahoo, R. S., Lamour, K. H., and Hausbeck, M. K. 2008. "Characterization of Phytophthora capsici causing foliar and pod blight of snap bean in Michigan." Plant Disease 92: 201–209.
French-Monar, R. D., Jones, J. B., and Roberts, P. D. 2006. "Characterization of natural populations of Phytophthora capsici associated with local weed populations in Florida vegetable farms." Plant Disease 90:345–350.
Roberts, P. D., Urs, R. R., French-Monar, R. D., Hoffine, M. S., Seijo,T. E., and McGovern, R. J. 2005. "Survival and recovery of Phytophthora capsici and oomycetes in tailwater and soil from vegetable fields in Florida." Annals of Applied Biology 146:351–359.
Ploetz, R., Heine, G., Haynes, J., and Watson, M. 2002. "An investigation into the biological attributes that may contribute to the importance of Phytophthora capsici as a vegetable pathogen in Florida." Annals of Applied Biology 140:140:61–67.