We may not realize it, but nearly all of us rely heavily on products derived from energy resources. Examples of such products include fossil fuel-based chemicals like plastics that are used almost everywhere in our daily lives. At present, most of our energy and petroleum-derived materials are made from nonrenewable resources, such as coal, petroleum, oil, and natural gas (fossil fuels), because they have been historically available as primary products from long-established industries. However, for several reasons, there is a growing urgency to produce energy and related bio-based products from renewable resources. Firstly, fossil fuels will be depleted very quickly because they cannot be replaced or produced as fast as they are being consumed. According to research done by the International Institute for Applied Systems Analysis, petroleum resources will last only another 70 years (Aguilera 2008). Eventually, when it becomes too costly to continue producing fossil fuels or when they become depleted completely, it will become necessary to use renewable resources as alternatives to fossil fuels. Secondly, deriving energy from fossil fuels has long-term negative impacts on the environment, such as greenhouse gas emissions, air pollution, and the associated effects of climate change (acid precipitation, ozone depletion, forest destruction, and the emission of radioactive substances) (Dincer 2000). Thirdly, the large amount of petroleum-derived materials (chemicals, plastics) that are made from fossil fuels further aggravates the depletion of nonrenewable energy resources. In addition, most petroleum-based plastics remain stable at ambient conditions for hundreds or thousands of years after being discarded, exacerbating the growing need for landfill space and adding to the environmental pollution caused by the toxic fumes these materials produce when incinerated. All these factors create a new impetus for the development of bio-based products (bioenergy and biomaterials) from renewable biomass resources. Many types of bio-based products can be produced or extracted from renewable biomass through biological, chemical, thermal, or mechanical treatments and used for power, heat, fuel, biogas, biochemicals, and biomaterials (Figure 1).
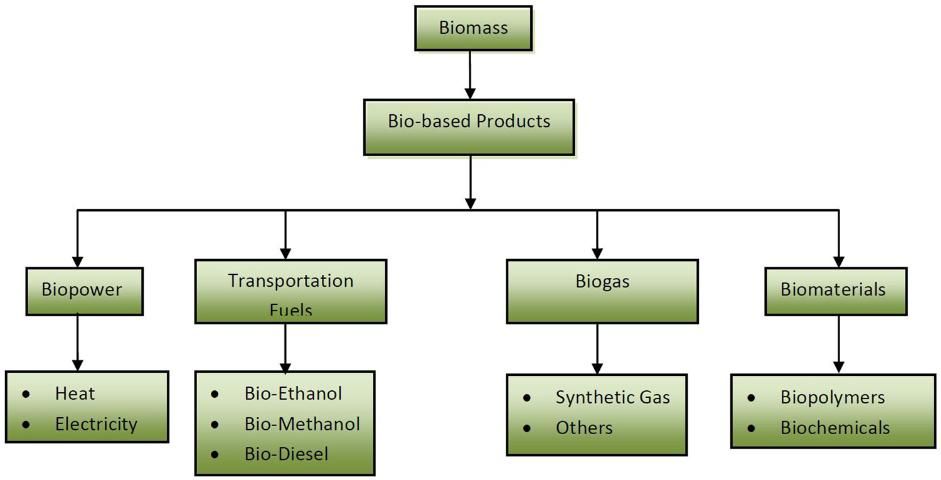
Many types of renewable resources, such as biomass, solar, wind, hydro/tidal, hydrogen, geothermal, and fuel cells, are capable of providing a portion of the energy we need. However, biomass can also produce a wide selection of bio-based by-products while producing renewable energy. Biomass is renewable biological materials, such as trees, plants, grasses, vegetables, algae, food wastes, animal manures, and other organic wastes. Each type of biomass has a different composition, such as sugar content, calorific value, moisture content, and ash content. By choosing different thermochemical, mechanical, or biological processes, such as combustion, hydrolysis, fermentation, gasification, or pyrolysis, biomass can be converted into a variety of products, including power, transportation fuels (ethanol, methanol, and biodiesel), biogas, biomaterials, and biochemicals.
Power from Biomass
Biomass has been an energy source for millennia. The simplest way to convert biomass to bioenergy is to burn it (firewood). The heat produced during burning can be used for cooking food and warming homes. In many third world developing countries, rural villagers still use biomass in their daily lives. Biomass has kept us warm for thousands of years through direct burning of wood in fireplaces and wood stoves, and it is now providing electricity when used as fuel in power plants. Biopower is heat or electric power made from biomass through various conversion processes. Most biopower plants use a direct or co-firing furnace to produce steam or power. Direct firing is the process of converting biomass into steam or heat without the addition of any other fuel. For example, paper mills are the largest producers of biomass power, and they co-generate electricity and steam through the burning of black liquor, a practice that has been ongoing since the 1930s. The black liquor, a waste liquid after the kraft pulping process of lignocelluloses (plant-based biomass mainly consisting of three main components: cellulose, hemicelluloses, and lignin), contains more than half the energy content of wood. Heat and electricity are generated by burning the black liquor (the mixture of lignin, chemicals, and water) at a solid content of 65%–80% while simultaneously recovering chemicals for pulp cooking (NETL 2017). Co-firing refers to mixing biomass with fossil fuel prior to injection into the same furnace, and it is an effective approach for reducing emissions (especially sulfur dioxide emissions) from a conventional power plant.
Transportation Fuels from Biomass
Transportation fuels are liquids (liquid or gas that can be stored in liquid form) with high energy densities. They usually consist of alkanes, alcohols, and esters, which can be easily vaporized (usually just by exposure to ambient air temperature and pressure) and burned cleanly within a heat engine. Traditional transportation fuels are classified as gasoline, diesel, or jet fuel. The United States still consumes more than one-third of the world's transportation energy because of its high per capita energy consumption (five times that of Japan and three times that of France). In the United States, transportation accounts for more than one-quarter of total energy consumption, and approximately 40% of this consumption comes from imported petroleum (Brown 2003). The use of alternative transportation fuels from biomass is critical to not only decrease dependence on foreign petroleum, but also to alleviate major environmental concerns associated with fossil fuel use. Current biofuel alternatives consist of bioethanol and methanol from plant biomass and biodiesel from vegetable oils and wastes (originally from oilseed crops). Ethanol and methanol are suitable for spark-ignition engines because of lower detonation requirements, which improve fuel economy and reduce engine damage. Biodiesel fuel is suitable for compression-ignition engines because of its short ignition delay and similar characteristics to conventional diesel fuel.
Bioethanol
Ethanol (also known as anhydrous alcohol) is a common type of chemical that is used intensively in the medical and food industries. More importantly, it can be used as fuel either alone or blended with gasoline in heavy machines and flexible fuel vehicles like new hybrids, which are growing in popularity. Ethanol is a clean fuel because the combustion of ethanol produces fewer pollutants than the combustion of fossil fuels. The limitations of bioethanol as fuel are as follows:
- It takes more fuel (volume and mass) to travel the same distance or do the same work because it has less energy content or is less energy dense (about two-thirds of gasoline).
- Ethanol has an affinity for attracting water and therefore has a greater tendency to corrode the combustion chamber, hoses, pipelines, and fuel systems than gasoline.
Bioethanol can be produced from a host of different natural biomass materials. These can be sugar, starch, or cellulose-based materials. Production of bioethanol from sugar (sugarcane or beets) is the easiest and most efficient process since sugar is readily fermented by yeast or bacteria. Starch-based materials, such as grains (corn, wheat, rice, etc.), potatoes, and sweet potatoes, are also suitable for bioethanol production. However, converting starch-based materials to ethanol requires an additional step in comparison with the sugar-based ethanol process. Starch-based materials (polysaccharides) are not capable of direct fermentation and must be hydrolyzed by enzymes to single sugars (glucose) before the fermentation process. About 3 kg of wheat can produce 1 L of absolute ethanol (Clark and Deswarte 2008).
There has been growing criticism that using food commodities (sugar, starch) for fuel will lead to higher prices for both food and fuel. This criticism might be addressed by using cellulose (instead of sugar or starch) to make bioethanol. This process does not use the edible part of the crop, but the cellulose part, which is inedible and is typically not used (stems, leaves, twigs, straw, grasses, etc.). Lignocellulosic materials, such as wood, agricultural grasses, and agricultural residues, can be used to produce ethanol as well. Using low-cost agricultural waste can minimize the impact of fuel production on the food supply and reduce production costs. However, converting cellulose-based materials to bioethanol is much more difficult than converting sugar- and starch-based materials. The process to convert cellulosic materials to ethanol usually includes three steps: pretreatment, hydrolysis, and fermentation. The pretreatment process breaks down the tangled network structure of lignocelluloses to make polysaccharides (cellulose and hemicelluloses) accessible for further hydrolysis to release the simple sugars, which are readily fermented by yeast or bacteria (Geddes et al. 2010). The cellulose-based bioethanol process has great potential because the lignocellulosic biomass is abundant and inexpensive, and technologies are developing rapidly to further reduce the processing cost and enhance the ethanol yield. The commercial production of bioethanol is from sugarcane in Brazil and from corn in the United States. Currently, there are no commercial cellulosic-based bioethanol facilities in the United States, but there are many cellulosic ethanol projects under development and construction, as shown in Figure 2 (ITEC 2017).
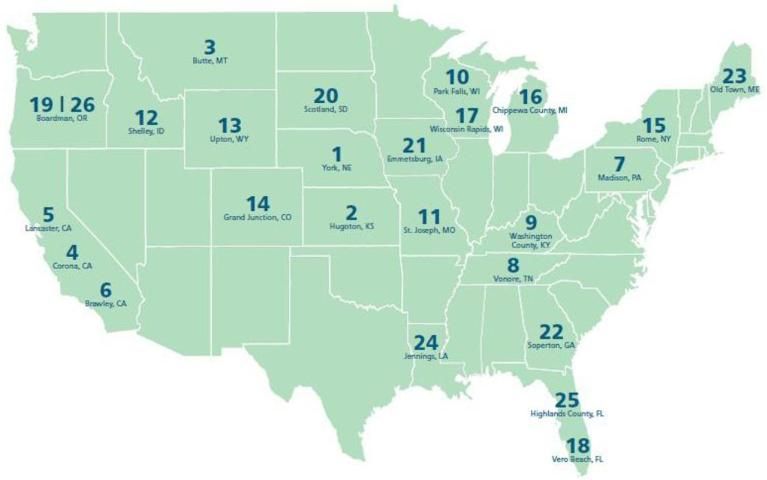
Credit: RFA 2009
Methanol
Methanol is the basic building block for many products, such as paints, furniture, auto parts, and plastics. As a fuel, methanol has similar chemical and physical properties as ethanol when it is used as transportation fuel because of its efficient combustion, ease of distribution, and wide availability. Methanol has added benefits as an alternative fuel, such as lower production cost compared to ethanol and low risk of flammability compared to gasoline. Methanol fuel has fewer gas emissions, such as carbon hydroxide and nitrogen oxide. It is widely used in racing or championship cars because it allows cars to run at extremely high compression ratios (more power) and is safer than gasoline because it burns cooler. The disadvantages of methanol are that it has lower energy density (half that of petroleum) and lower vapor pressure, similar to ethanol. Therefore, methanol and ethanol evaporate slowly in low temperatures and can cause problems when starting a car in cold temperatures. Biomethanol is more toxic to the human body than ethanol and is incompatible with current engine fuel systems. However, methanol can be added to gasoline in small amounts (< 10%), which helps reduce cost and requires no modification to fuel systems (Brown 2003). Ethanol can also be added to gasoline in an amount less than 10%.
Methanol is commonly produced from natural gas in most of the world, but China produces it from coal on a large scale. Methanol can be obtained from a broad range of waste biomass materials through fermentation or thermochemical processes like pyrolysis, which convert it to biogas. Methanol can be used as transportation fuel in three ways: blending with gasoline, producing dimethyl ether (DME) as a diesel replacement, or participating in the biodiesel production process as a catalyst. For future transportation energy needs, automotive companies are working on hybrid electric vehicles, but one current limitation is adequate battery capacity. Methanol can be used in fuel cells to produce adequate electricity for these types of applications, but this technology is still in the research and development stage (Methanol Institute 2011).
Biodiesel
Biodiesel fuel has been around for several years now in a number of public transportation systems. For example, hundreds of US school bus fleets are using B20 (20% biodiesel blended with 80% diesel), Seattle City Light is buying low-emission biodiesel fuel for use in state ferries and in Metro buses, and buses in Gainesville, Florida, have been powered by biodiesel since 2008. Biodiesel fuel is a clean-burning alternative fuel produced from renewable biomass resources. Generally, biodiesel (methyl/ethyl ester) fuels are produced by reacting vegetable oil (extracted from oilseed crops) or animal fat with methanol/ethanol and catalyzed by caustic soda. This process is called transesterification (ESRU 2011). Both vegetable oil and animal fat produce biodiesel fuels that require little, if any, modification to existing diesel engines. Biodiesel has some advantages over conventional petroleum diesel. Vegetable oil-based and animal fat-based biodiesel fuels ignite more easily than regular diesel fuel. Also, biodiesel's lubricity is far superior to the low-sulfur regular diesel currently on the market. This is important because fuel injectors and some types of fuel pumps rely on fuel for lubrication. Moreover, biodiesel has high oxygen content, which facilitates combustion and reduces waste gas emissions (carbon monoxide and hydrocarbon) and particulates. It is also considered environmentally friendly. However, biodiesel use has some limitations. First, biodiesel has 9%–13% lower energy content than petroleum diesel; therefore, slightly lower power and torque and higher fuel consumption can be expected compared to petroleum diesel. Second, biodiesel starts to form wax crystals and has flow problems at low temperatures (< 40°F), which limit the use of biodiesel in cold weather (Brown 2003). Typically, as the weather cools in northern climates, less biodiesel (< 50%) is used.
Biodiesel production capacity has been growing rapidly, with an average annual growth rate from 2002–2006 of more than 40% (REN21 2008). The most important reason for the rapid growth in biodiesel production is that the US government is encouraging biodiesel production by reducing taxes and offering grants. Currently, soybean oil is the dominant biomass source for biodiesel production. The cost of biodiesel production from virgin vegetable oil is predicted to be approximately three times greater than regular diesel, and the price is expected to further increase to $1.00 per litre as the demand for virgin vegetable oil increases as both a food crop and source of biomass for biodiesel production (Global bioenergy 2009). Therefore, at present, animal fats and low-grade greases have attracted more and more attention because of the increasing price of vegetable oils. Animal fats can be extracted from waste fat as lard and tallow from the meat processing industry. Yellow grease, another cheap resource from which to make biodiesel, is a form of low-grade oil that includes used frying oil or oil derived from animal fats.
Biogas from Biomass
Biomass (wood, algae, bagasse, sawdust, coconut shell, etc.) can be converted into a synthetic gas (syngas) through a gasification process (pyrolysis) with about one-third of the oxygen necessary for complete combustion. The gas usually includes carbon monoxide and hydrogen and can be directly used in place of gasoline in vehicles with a filtering and cooling treatment. The UF/IFAS Extension Taylor County office has demonstrated the use of a wood gasification system installed on a truck that was driven 2,000 miles for $20 in fuel cost using an average of 1 lb of wood per mile at highway speeds. Char is a by-product of the gasification process made up of water vapor and ash. The same syngas in stationary production systems can be used to generate power for irrigation or steam as well as to produce chemicals (Evans 2011). Biomass can also be converted into bio-oil, (lower temperature) biochar, and syngas through pyrolysis in the absence of oxygen. Bio-oil can be used directly as a clear fuel or converted to other chemicals. In addition, biogas can be produced from biomass waste with high moisture content (food processing waste, animal manure, algae). Another technology used to produce biogas (primarily methane mixed with carbon dioxide) is a process called anaerobic digestion, which operates in complete absence of oxygen. The primary components of biogas through anaerobic digestion consist of methane and carbon dioxide and small quantities of nitrogen, hydrogen, hydrogen sulphide, and even oxygen. Biogas collected on site is directly used in individual farms or homes for electricity and heat generation (ABC 2017).
Biomaterials and Biochemicals from Biomass
Biomass can not only provide fuel and energy, but it also can be a source for the production of countless biomaterials and biochemicals that can be used as, among other things, paints, detergents, industrial adhesives, bioplastics, and composite materials. Conventional biomaterials like wood and linen have been used to build homes and make clothes since the earliest times. In the textile industry, half of all fiber comes from natural materials such as ramie, cotton, wool, and flax. In the paper industry, biomass in the form of pulpwood (lignocelluloses) is used to make paper and other packaging materials. In the pharmaceutical industry, certain drugs and antibiotics can only be made from natural biomass materials through fermentation. In the plastics industry, the continued depletion of fossil fuel and the tremendous amount of plastic waste accumulated in the environment has drawn attention back to bioplastics (biopolymers), which have experienced rapid growth in the last few decades. There are numerous ways to obtain chemicals from biomass. Natural chemicals, such as polysaccharides, sucrose, triglycerides, and natural rubber, can only be separated by physical methods. Biochemicals such as cellulose and starch-derivative chemicals, glucose, glycerol, citric acid, and lactic acid can be obtained by chemical modifications and fermentation. The basic building blocks of petroleum-based chemicals can be produced indirectly from the synthetic gas of biomass gasification or its fermentation products, such as ethanol, sorbitol, methanol, amines, and succinic acid (Brown 2003).
Conclusion
Bio-based products hold great potential to resolve the current energy and environmental crises. However, the choice to use biomass for power, fuel, and biomaterials depends on a variety of factors, such as availability, public policy, cost of biomass, capital cost of process equipment and facilities, and markets for alternative energy and materials. The development of advanced conversion technologies suitable for different types of biomass is likely to make bio-based products (energy and materials) competitive with petroleum-based products. In addition, these challenges and solutions can be addressed and advanced by progressive federal and state policies, technological progress, and social awareness of environmental problems.
References
ABC (American Biogas Council). 2017. "What is anaerobic digestion?" Accessed October 2017. https://www.americanbiogascouncil.org/biogas_what.asp
Aguilera, R. F. 2008. "How Long Will Petroleum Resources Last?" EU Energy Policy (blog). Accessed July 2011. http://www.energypolicyblog.com/2008/11/23/how-long-will-petroleum-resources-last/.
Brown, R. 2003. Biorenewable Resources: Engineering New Products from Agriculture. Hoboken, NJ: Wiley-Blackwell.
Clark, J. H., and F. Deswarte. 2008. Introduction to Chemicals from Biomass. Hoboken, NJ: Wiley-Blackwell.
Dincer, I. 2000. "Renewable Energy and Sustainable Development: A Crucial Review." Renewable & Sustainable Energy Reviews 4(2): 157–175.
ESRU (Energy Systems Research Unit), University of Strathclyde. 2011. "What Is Biodiesel?" http://www.esru.strath.ac.uk/EandE/Web_sites/02-03/biofuels/what_biodiesel.htm.
Evans, S.D. 2011. "Biomass Gasification and Syngas." http://ezinearticles.com/?Biomass-Gasification-and-Syngas&id=1933540.
Geddes, C. C., J. J. Peterson, C. Roslander, G. Zacchi, M. T. Mullinnix, K. T. Shanmugam, and L. O. Ingram. 2010. "Optimizing the Saccharification of Sugar Cane Bagasse Using Dilute Phosphoric Acid Followed by Fungal Cellulases." Bioresource Technology 101(6): 1851–1857.
Global bioenergy 2007. "Cost of biodiesel production". Accessed October 2017. http://www.globalbioenergy.org/uploads/media/0305_Duncan_-_Cost-of-biodiesel-production.pdf.
ITEC 2017. "Cellulosic Ethanol". Accessed October 2017. http://www.itecref.com/cellulosic-ethanol.html
Methanol Institute. 2011. "Methanol Basics." http://www.methanol.org/Methanol-Basics.aspx.
NETL (National energy technology laboratory), "Black liquor gasification." Accessed October 2017. https://www.netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/blackliquor
REN21 (Renewable Energy Policy Network for the 21st Century). 2008. "Renewables 2007 Global Status Report." Accessed June 25, 2011. http://www.martinot.info/RE2007_Global_Status_Report.pdf.