Fertilization is used in land-based agriculture to increase productivity of a field. One can also increase the productivity of a pond by adding fertilizer. If a fish species which consumes small natural foods is grown, such as the bluegill or golden shiner, then pond fertilization can increase the production of these fish. Fertilizers provide nutrients to microscopic plants known as algae. These algae are eaten by microscopic animals (zooplankton) and insects, both of which serve as food for small fish. These small fish are in turn eaten by larger predatory fish. Fertilization of a pond which has both bluegill and largemouth bass, will thus result in greater numbers of larger bass because of the increased number of bluegill for them to eat. With proper fishing, the pond will also produce greater numbers of larger bluegill. Fertilized ponds can have fish yields three to four times over that of unfertilized ponds.
Another application of fertilization is to prepare an aquaculture pond for stocking of fish fry from a hatchery. Many fish fry (newly hatched fish) require live food in their early stages of development. Fertilization of a pond is one way to increase the amount of small plants and animals available to the fry.
In freshwater ponds, the most needed nutrient is usually phosphorus, and additions of phosphorus alone will add to the production of the pond. However, by adding some nitrogen as well, you will obtain a better result. Fertilizer manufacturers are required to provide you with the grade of their product by including its N–P–K (nitrogen– phosphorus–potassium) value on the label. Each value represents the percent, by weight, of that nutrient in the fertilizer. For example, a 10–34–0 fertilizer contains 10% nitrogen, 34% phosphorus (as phosphoric acid), and no potassium.
Fertilizer is available in a liquid or granular form. Liquids are best for pond applications because they promote rapid growth of algae and because smaller applications can be used which may reduce the cost of pond fertilization. As mentioned above, phosphorus is the most needed nutrient in ponds, but it is relatively insoluble in water. It takes a long time for granular fertilizer to be effective, and if the phosphorus portion sinks to the bottom, it is tied up chemically in the mud.
Liquid fertilizers generally have a high percentage of phosphorus, and this phosphorus is in a form that is immediately available to the algae. Effects on the algae populations can be seen within days of application with a liquid fertilizer. For these reasons, it is recommended to use liquid fertilizers whenever possible.
Most liquid fertilizers have a similar N–P–K value (see Table 1), so application rates are the same. With granular fertilizers, the application rates will vary depending on the N–P–K value of the fertilizer that you are using.
When to Fertilize
- In Florida, the climate varies a great deal from the northern part of the state to the southern-most part of the state, so it is not possible to recommend a specific time of the year to begin a pond fertilization program. A simple rule of thumb which you can use, however, is to start fertilizing as soon as the water temperature is above 65°F. In extreme south Florida, this may translate into a year-round fertilization schedule.
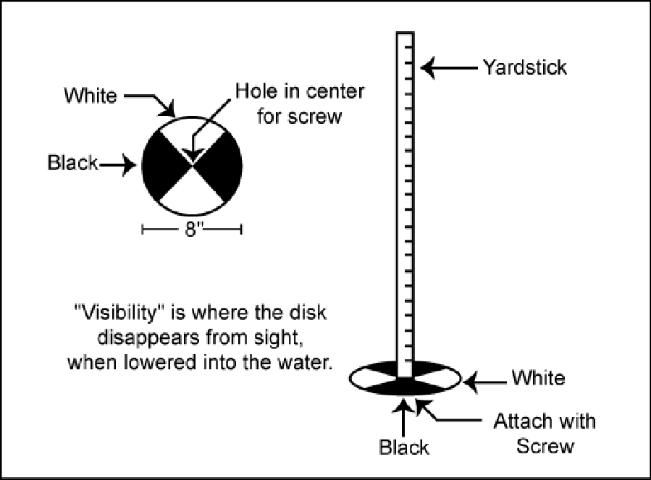
- Repeated applications should be done on a monthly basis, or whenever the water clears enough so that a white (or black and white) disk attached to a yardstick is visible to a depth of 18 inches or more (Figure 1).
- Dividing the monthly application into two smaller applications, every two weeks, will produce better results.
- Continue applications until the water temperature drops below 70°F (usually November).
Credit: UF/IFAS
How to Apply Fertilizer
Liquid fertilizer is heavier than water and will sink to the bottom of your pond unless first diluted with water. There are many ways to accomplish this, such as:
- Dripping the fertilizer into the propeller wash of a motor boat. The propeller will mix the fertilizer into the water.
- Using a garden hose sprayer to apply the fertilizer to the surface of the pond.
- Diluting the fertilizer with water in a wash tub or large bucket before adding it to the pond.
The size of the pond being fertilized and equipment available will determine how you will dilute the fertilizer. The important thing to remember is to dilute it as much as possible before applying it, and to distribute the liquid as evenly as possible across the entire surface of the pond.
Granular fertilizers should be placed on a platform, positioned one foot below the surface, and allowed to dissolve. If granular fertilizers are simply broadcast across the pond's surface, they will sink to the bottom, where most of the phosphorus will be tied up chemically in the mud.
Often it is difficult to get a response from the algae with fertilization and the following are a few possible reasons for this:
- Muddy water—If the water is too muddy, the sun's light cannot penetrate into the water well enough to support the algae. The turbidity of the water must first be cleared before a pond can be fertilized.
- Aquatic weeds—If the pond has an abundance of aquatic plants, do not apply fertilizer. The plants will out compete the algae for the nutrients, and you will just end up with more weeds. Fertilization programs should be started early in the year before plants begin to grow.
- Water exchange—Fertilizers will not work in ponds which are continuously and excessively exchanging water. Spring-fed ponds are an example of poor candidates for fertilization.
- Low alkalinity—The solubility of phosphorus is lower at low alkalinities, and extremely acidic waters may not respond to fertilization. For good results, the alkalinity should be at least 20 parts per million. Liming of ponds with lower alkalinities will usually relieve this problem.
Measuring the Visibility of a Pond
A simple tool to measure the visibility of a pond for fertilization purposes can be made with materials that are found around most homes. An 8-inch diameter white disk is attached to the end of a yardstick. The disk can be cut from scrap pieces of sheet metal or plastic. Figure 1 gives a fairly self-explanatory diagram of how to make one. By lowering the disk straight down into your pond, you can measure the point at which it disappears. This is the visibility or water clarity. For most situations, a visibility between 12 and 18 inches is desired. Anything less than 12 inches indicates that too much fertilization is likely to be taking place, and a visibility of more than 18 inches indicates that fertilization is needed.
Many ponds in Florida are naturally fertile and do not have to be fertilized because of the nutrient-rich soils in which they occur. Also ponds in which the fish are fed or which receive runoff water that is rich in nutrients generally do not require fertilization. Pond owners should be committed to a long-term management program before fertilization begins. One-time or haphazard fertilization is uneconomical and often causes aquatic weed problems.