Introduction
Production of ornamental fish in Florida accounts for approximately 80–90 percent of all aquarium fish raised in the United States. Florida's farmers breed and raise hundreds of species of aquarium fish, using a variety of techniques. Some species will spawn after simple environmental changes have been made, such as changes in water temperature, pH, or conductivity. Other species require more advanced methods, including administration of hormone products by injection ("induced spawning").
One of these products, Ovaprim (Western Chemical, Inc., Ferndale, WA), has been used worldwide for over a decade as a spawning aid for many different species of fish. Ovaprim was available in the United States for a number of years for commercial ornamental fish breeders only as an FDA Investigational New Animal Drug (INAD) administered through the University of Florida's Tropical Aquaculture Laboratory.
In March of 2009, Ovaprim became the first new animal drug added to the FDA Index of Legally Marketed Unapproved New Animal Drugs for Minor Species (i.e., the Index). This means ornamental fish farmers can now legally purchase Ovaprim directly from the manufacturer. Under the Index, Ovaprim is intended for use as a spawning aid for ornamental fish broodstock.
An understanding of Ovaprim's mode of action and considerations for its use are critical for successful use of this drug during ornamental fish production.
How is spawning triggered naturally?
Spawning, the release of gametes (mature oocytes/eggs by the female or of sperm/milt by the male) from the gonads (ovaries or testes), is the final event in the reproductive cycle (Rottmann et al. 1991a), and is the result of complex interactions between a variety of hormones and different tissues/organs in the fish's body.
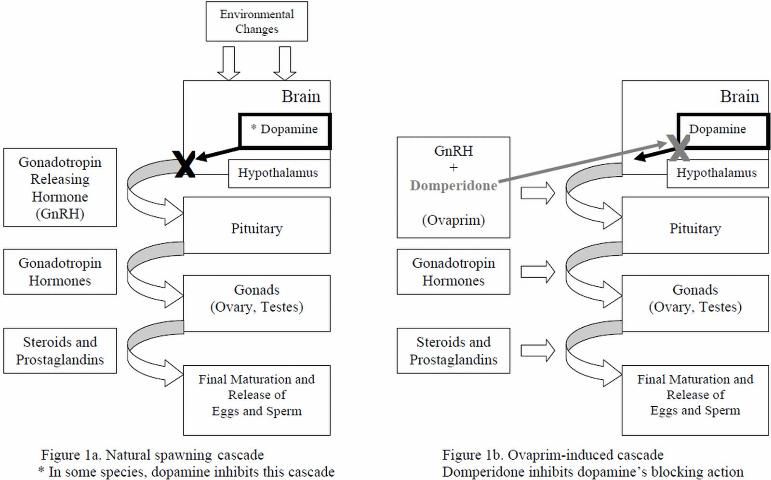
The following description is a very simplified version of major events (Rottmann et al. 1991a; Figure 1a) that occur during natural spawning. Environmental and internal factors (which differ from species to species) trigger a hormone cascade leading to egg maturation and release in females, and sperm maturation and release in males. First, a hormone called "gonadotropin-releasing hormone" (GnRH) is released from the hypothalamus portion of the brain and travels to the pituitary gland, which then releases gonadotropin hormones (especially GtH-II). The gonadotropin hormones/GtH-II travel to the ovaries and testes and stimulate them to produce steroids and prostaglandins, hormones that act directly on the gonads to cause final maturation and release of oocytes ("eggs") in females (ovulation), and the release of sperm in males (spermiation) (Rottmann et al. 1991a; Evans and Claiborne 2006). In some species of fish, a compound called dopamine naturally blocks this hormone cascade and inhibits egg and sperm production under certain conditions, for example, exposure to major stressors.
What is Ovaprim?
Ovaprim is a two-part drug consisting of 1) a shorter, synthetic version of GnRH and 2) domperidone. Ovaprim is marketed ready to inject in a liquid form.
In the ornamental fish industry, Ovaprim is used as a spawning aid to induce ovulation (release of mature oocytes/eggs) and spermiation (release of milt/sperm) in mature, properly conditioned brood-fish. Ovaprim is especially useful for species for which natural spawning in captivity is difficult to induce.
NOTE: Use of Ovaprim does not guarantee successful fertilization, development, and hatch of fry. Overall good husbandry and hatchery management, including good genetics, proper nutrition, environment (including water quality), substrates, social structure, and other factors will affect egg and milt quality, egg hatch, and fry survival.
How does Ovaprim work?
For some ornamental species, determining which natural environmental cues will lead to ovulation and spermiation has been difficult. Ovaprim's synthetic GnRH closely resembles that of naturally occurring GnRHs and, in many species, is actually more potent (Figure 1b). When injected into the body cavity (coelom or abdomen) or muscle of receptive, mature, conditioned fish, the synthetic GnRH travels from the injection site through the blood to activation sites in the pituitary gland. Ovaprim initiates the reproductive cascade and eliminates the need for a natural trigger. Domperidone, the other active component of Ovaprim, helps block the inhibitory effects of dopamine. Domperidone, therefore, is very important for induced spawning of species for which the reproductive cascade would be stopped because of stressors that lead to dopamine release, because dopamine will block GnRH activity.
What do I need to know before I use it?
Ovaprim is intended for use as a spawning aid in reproductively mature, conditioned fish. The aquaculturist should know optimal water quality for both conditioning and spawning, including temperature; the approximate size and/or age of maturity in both males and females for a given species; appearance of mature, conditioned males and females; and methods for assessing gonadal development (for both ovaries and testes). Conditioning diet, although difficult to optimize for some species due to uncertainties, should be as complete and abundant as possible.
How do I check my fish for maturity?
For many fish species, sex determination is based on the differences in coloration or body shape. In species where males and females look similar, one crude method that is often useful to determine sex of the fish is based on the larger volume of the eggs in conditioned females vs. sperm in males. Females may become more robust around the coelomic cavity (from enlarged ovaries due to egg yolk accumulation in late stages). In some cases, more robust fish may in fact be fat males. Consequently, careful collection and examination of eggs from the ovary (or sperm from the testes) is highly recommended for a more accurate assessment.
Catheterization and surgical biopsy are two different accurate methods that can be used to determine sex of the fish and maturity of the eggs (or sperm). For catheterization, a small-bore rubber tube (i.e., catheter) is placed into the genital pore/vent/opening and is rotated gently through the oviduct into the ovary. Once the tube is properly inserted, a sample of eggs may be extracted by gentle suction or by removing the catheter while blocking the open end. The eggs can then be "staged" (their developmental status determined) using a microscope (Rottmann et al. 1991c). For some species of fish, a more complicated, surgical approach via incision into the body cavity is necessary; however, this should be performed only by a qualified individual. Eggs that are in a late stage of development are uniform in size and large (yolk-filled). They have a germinal vesicle (nucleus) that has migrated away from the center of the egg (Rottmann et al. 1991c). Mature, conditioned males will often release milt during handling or after gentle pressure is applied to their coelomic cavity.
Ovaprim should only be used once broodstock are determined to be properly conditioned and sexually mature with late stage gonads.
What supplies do I need to use Ovaprim?
-
Tank(s) with aerated water for sedation
-
Tank(s) with aerated water for recovery
-
Sedative
-
Syringes with permanently attached needles or with luer lock tips and separate needles (22–28 gauge)
-
For smaller fish (100 grams or less), use of microliter (μL ) syringes with smaller gradations (typically 100 μL volume divided into 1 μL increments) and with screw-on needles will make administration simpler.
What else should I know before I use Ovaprim?
Because Ovaprim is a viscous liquid, the use of a syringe with a luer lock tip or permanently attached needle is recommended. Slip-on type needles may eject from the pressure, so if you use them, be sure they are seated on the syringe tightly.
To avoid introduction of bacteria or other pathogens via injection, use only sterile needles and syringes to withdraw Ovaprim from the bottle, and to inject the fish. After withdrawing the correct amount required for the specific fish, carefully point the syringe and needle upward and depress the syringe to remove any excess air.
How do I determine the right dose?
For many species, a single dose at 0.5 mL Ovaprim per kilogram fish body weight, which is equal to 0.5 μL/gram body weight, is required. There are 1000 μL per 1 mL. To calculate the volume of Ovaprim needed, determine the weight of your fish and use the appropriate formula below:
(Weight of fish in kg) x 0.5 mL/kg = mL of Ovaprim required.
(Weight of fish in lbs) x (kg/2.2 lbs) x 0.5 mL/kg = mL of Ovaprim required.
(Weight of fish in grams) x 0.5 μL/gram = μL of Ovaprim required.
For example, if your brood fish weighs 70 grams, then:
70 grams x 0.5 μL/gram = 35 μL of Ovaprim
Additionally, species and environmental differences may warrant a slightly different regimen. Although a single dose works well for many species, a split dose, may improve success. Producers may want to consider a split dose if—assuming all other considerations have been examined—repeated attempts of a single dose are unsuccessful. In this two-dose scheme, for warm water species, the first (or "priming") dose is administered using 10% of the total volume; the second dose is administered at least 6 hrs later using the remaining 90% volume.
Where do I inject the fish?
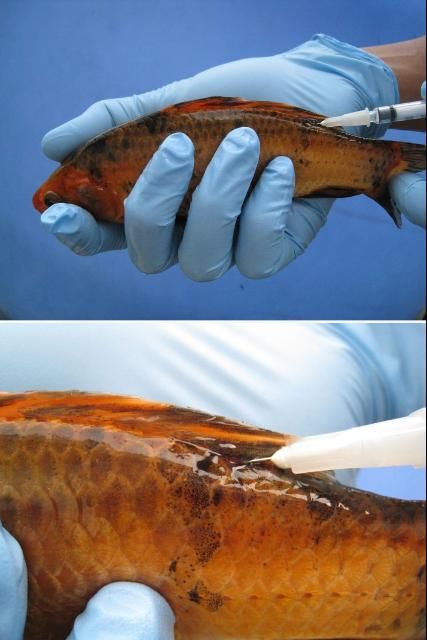
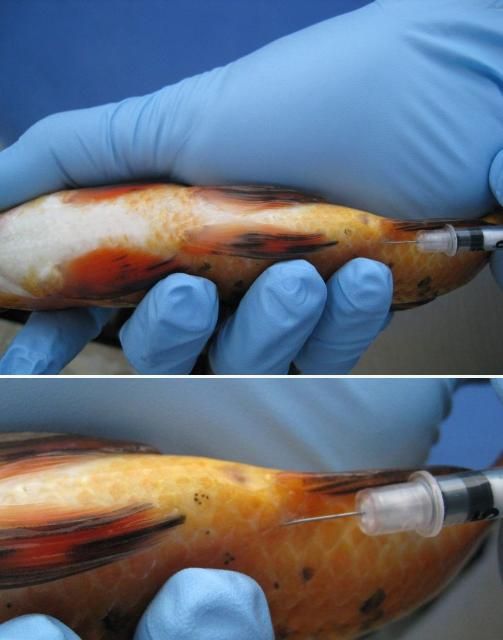
First, immobilize the fish using the correct dose of an anesthetic (e.g., tricaine methanesulfonate ["MS-222"] or metomidate). Fish should be injected either in the muscle (IM) or in the body cavity (IP or ICe; i.e., in the "peritoneal cavity" or coelomic cavity) (see Figures 2 and 3 ). If you are injecting into the muscle (IM), a good area in many species is the dorsal (top) area of the fish, immediately behind or near the dorsal fin. If you are following a two-injection protocol, site the two injections on opposite sides of the fish. If you are injecting the fish into the body cavity (IP or ICe), turn it so that its ventral surface (belly) is up and its posterior (back ) end is slightly elevated. Inject into the body cavity in an area that is forward of but slightly off center to the anus or vent, and be sure not to go too deep. If you make the injection too deep, you may accidentally inject into internal organs rather than into the body cavity itself. This positioning will help minimize the likelihood of injection into and damage to the liver, intestine, or other organs.
How will I know when the fish are ready to release their eggs or milt?
The length of time after the Ovaprim injection when final maturation, ovulation, and spermiation will occur depends upon the species, the water temperature, and other factors. For some warm-water species, these events may occur as soon as 4 hours after the final injection, with others taking up to 12 hours. Consequently, fish should be inspected periodically after treatment. However, because time to ovulation or spermiation may vary, handling and disturbance should be minimized. Signs include a swelling or "softening" of the abdomen (coelom); presence of eggs in the water; easy expression of eggs or milt from broodstock with gentle pressure; or evidence of spawning behavior with mixed sex populations. In some groups, males may respond more rapidly than females.
Should I "strip" the fish or let them spawn naturally?
The decision whether to express the gametes (eggs and sperm) manually from the fish (i.e., to "strip" the fish) or to allow the fish to spawn on their own will depend upon a number of factors, including the species biology, number of males and females, availability of facility space and equipment, and the production scheme.
For some species and for some production schemes, stripping all males and females is preferred to minimize space and equipment requirements and to maximize production efficiency and synchronization of spawning. Manually stripping fish will often increase fertilization rates, allow for more uniform dispersal of fertilized eggs among production units, and prevent the brood fish from eating the eggs.
However, in some situations, manual stripping is not necessary or desirable. For some species, manual stripping is contraindicated because of biological considerations, including susceptibility to injury from handling, size, need for direct parental involvement, or other factors. In these situations, if there is ample system space, equipment, including proper spawning substrate, time, and adequate sex ratios, or if spawning is not well understood for a given species, aquaculturists may choose to allow the fish to spawn naturally.
If you are not familiar with how to manually strip fish for spawning, see Rottmann et al. (1991f).
For what species has use of Ovaprim been successful?
Ovaprim has been used successfully in many different families and species of ornamental fish, including members of the family Cyprinidae (koi, goldfish, barbs, freshwater sharks), Characidae (pacu), Cobitiidae (loaches), different species of catfish (Order Siluriformes), and Helostomatidae (kissing gourami), in addition to other fish families and species.
What are some cases in which the use of Ovaprim may not lead to the release of eggs or milt?
If you take into account all the considerations described in the sections above and follow the instructions, the chances of successful release of eggs and milt are greatly enhanced. However, there are a number of factors that may a) prevent Ovaprim from working in a given situation; b) prevent successful stripping or spawning; or c) lead to complications (including disease or mortality) post-treatment. Some of these are listed below.
Fish-related considerations:
-
Fish are not sexually mature and/or are not properly conditioned.
-
Fish have a subclinical disease (bacterial, parasitic, viral infection)
-
Species may use a different form of GnRH
-
Species may have a different initiating reproductive cascade and/or other inhibitors besides dopamine
-
Females may have blockage in the oviduct, preventing expression of eggs
-
Males testes may not have proper anatomy to permit manual stripping
Environment-related considerations:
-
Incorrect water temperature
-
Low dissolved oxygen
-
High ammonia
-
High nitrite
-
Improper pH
-
Improper conductivity or salinity
-
Improper hardness or alkalinity
-
Improper photoperiod
Procedure-related considerations:
-
Incorrect dosage
-
Fish handling is excessive or rough
-
Injection made in the wrong site, or in a less than optimal site
-
Ovaprim has not been stored properly or is used after expiration date
-
Non-sterile needles or syringes have been used, leading to infection
How should I store Ovaprim?
Store below 77°F (25°C) and protect from sunlight and sources of heat.
How do I dispose of any unused product?
Contact your State Environmental Control Agency, or the Hazardous Waste Representative at the nearest EPA Regional Office for guidance pertaining to disposal of unused product.
Are there any human health or safety concerns with use of Ovaprim?
As with all over-the-counter drugs, Ovaprim poses minimal risk and is safe and effective when used as directed on the label. Use in a well ventilated area. Wear gloves, goggles, and suitable protective clothing.
Ovaprim is not intended for use in humans. Keep out of the reach of children. Avoid accidental contact (such as through inhalation, ingestion, or eye or skin contact) or self-injection. Seek medical advice immediately if you have concerns about inhalation, ingestion, eye or skin contact, or self-injection.
If you have any questions or problems concerning the use of Ovaprim, contact the manufacturer and/or a knowledgeable production specialist immediately.
Summary
For some species of ornamental fish, successful reproduction requires the use of hormone products. Ovaprim is one such drug and consists of a synthetic GnRH and domperidone in a liquid propylene glycol carrier. Ovaprim is the first new animal drug added to the FDA Index and can now be used as an over-the-counter injectable spawning aid in ornamental finfish.
Ovaprim acts by stimulating a hormone cascade that results, ultimately, in the release of eggs and sperm. Ovaprim will work successfully only in fish that are sexually mature, properly conditioned, and in the final stage of maturation.
Proper use of Ovaprim will help facilitate synchronized and more efficient spawning of ornamental species, including those for which the natural environmental cues needed for successful spawning have not been determined.
References and Further Reading
Cheah, M.S.H., and C.L. Lee. 2000. Induced ovulation of the Australian eel-tailed catfish Neosiluris ater (Perugia) with Ovaprim. Asian Fisheries Science 13:87–96.
Evans, D.H., and J.B. Claiborne. 2006. The physiology of fishes. Third edition. Taylor and Francis, CRC Press, 601 pp.
FDA-CVM MUMS Drug Indexing (accessed October 13, 2009) http://www.fda.gov/AnimalVeterinary/DevelopmentApprovalProcess/MinorUseMinorSpecies/ucm070206.htm
FDA-CVM MUMS Index List (accessed October 13, 2009) http://www.fda.gov/AnimalVeterinary/DevelopmentApprovalProcess/MinorUseMinorSpecies/ucm125452.htm
Haniffa, M.A., P.S. Allen Benziger, A.J. Arockiaraj, M. Nagarajan, and P. Siby. 2007. Breeding behaviour and embryonic development of koi carp (Cyprinus carpio). Taiwania 52(1):93–99.
Hill, J.E., J.D. Baldwin, J.S. Graves, R. Leonard, J.F.F. Powell, and C.A. Watson. 2005. Preliminary observations of topical gill application of reproductive hormones for induced spawning of a tropical ornamental fish. North American Journal of Aquaculture 67:7–9.
Hill, J.E., K.H. Kilgore, D.B. Pouder, J.F.F. Powell, C.A. Watson, and R.P.E. Yanong. 2009. Survey of OvaprimTM use as a spawning aid in ornamental fishes in the United States as administered through the University of Florida Tropical Aquaculture Laboratory (2005–2007). North American Journal of Aquaculture 71(3): 206–209.
Ovaprim Freedom of Information Summary (accessed November 12 2009) http://www.fda.gov/AnimalVeterinary/DevelopmentApprovalProcess/MinorUseMinorSpecies/ucm125475.htm
Ovaprim Label (accessed November 12, 2009) http://www.fda.gov/AnimalVeterinary/DevelopmentApprovalProcess/MinorUseMinorSpecies/ucm125478.htm
Powell, J.F.F., S.L. Krueckl, P.M. Collins, and N.M. Sherwood. 1996. Molecular forms of GnRH in three model fishes: rockfish, medaka and zebrafish. Journal of Endocrinology 150:17–23.
Powell, J.F.F., J. Brackett, and J.A. Battaglia. 1998. Induced and synchronized spawning of captive broodstock using Ovaplant and Ovaprim. Bulletin of the Aquaculture Association of Canada 3:14–18.
Rottmann, R.W., Shireman, J.V., and F.A. Chapman. 1991a. SRAC 0421 Introduction to Hormone-Induced Spawning of Fish. Southern Regional Aquaculture Center. USDA
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991b.SRAC 0422 Capturing, Handling, Transporting, Injecting and Holding Brood Fish. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., Shireman, J.V., and F.A. Chapman. 1991c. SRAC 0423 Determining Sexual Maturity of Broodstock for Induced Spawning of Fish. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991d. SRAC 0424 Hormonal Control of Reproduction in Fish for Induced Spawning. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991e. SRAC 0425 Hormone Preparation, Dosage Calculation, and Injection Techniques. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991f. SRAC 0426 Techniques for Taking and Fertilizing the Spawn of Fish. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991g. SRAC 0427 Induction and Verification of Triploidy in Fish. Southern Regional Aquaculture Center. USDA.
Sahoo, S.K., S.S. Giri, and A.K. Sahu. 2005. Induced spawning of Asian catfish, Clarias batrachus (Linn.): effect of various latency periods and sGnRHa and domperidone doses on spawning performance and egg quality. Aquaculture Research 36:1273–1278.
Sarkar, U.K., P.K. Deepak, R.S. Negi, S. Singh, and D. Kapoor. 2006. Captive breeding of endangered fish Chitala chitala (Hamilton-Buchanan) for species conservation and sustainable utilization. Biodiversity and Conservation. 15:3579–3589.
Western Chemical, Inc., Ferndale, WA (accessed November 12, 2009) http://www.wchemical.com/ovaprim-ovammmlu010.html