The World Health Organization (WHO) defines probiotics as "live microorganisms which, when administered in adequate amounts, confer a health benefit on the host" (Hill et al. 2014). In the United States, probiotics are considered dietary supplements, not food or drugs, and thus are not specifically regulated by the Food and Drug Administration (FDA). As dietary supplements, some products containing live microorganisms have been marketed as probiotics without having undergone the testing needed to confirm their health benefits. It is recommended that only microbial species that have been shown to confer health benefits in well-designed, controlled studies should be considered probiotics (Hill et al. 2014). Live cultures that are used in the fermentation of foods (e.g., yogurt) and that have no demonstrated health benefits are not considered probiotics.
Probiotics are beneficial for gastrointestinal wellness, immunity, and a variety of other health outcomes (Sanchez et al. 2016). There are many probiotic supplements available in the marketplace and choosing a supplement can be challenging. This review provides a summary of the health benefits of probiotics that are backed by high levels of scientific evidence.
Many bacteria and other microorganisms have been tested for their beneficial effects on health. It is important to note that these microorganisms may differ in their health effects, so each potential probiotic needs to be tested to determine if it exerts a specific health benefit. Research carried out in animals alone cannot be used to support recommendations for humans. Human research studies need to be completed on all potential probiotics. Furthermore, a positive health effect from a single human research study is not sufficient to confirm the health benefit of a probiotic. A number of well-designed studies demonstrating similar positive findings provide the strongest evidence that a probiotic is effective.
A high level of scientific evidence is considered to be a systematic review with meta-analysis. A systematic review is a structured search and review of all studies to date examining a specific health benefit of a probiotic. In health research, it is common to pool and statistically analyze the results of studies, a technique known as meta-analysis. Positive findings of a systematic review with meta-analysis strongly suggests efficacy, i.e., the probiotic exerts a specific health benefit. It has been suggested that systematic reviews should be conducted on studies of a single species (McFarland 2016), although most reviews to date have combined different species. Many of the health benefits of probiotics described in this review have this strong level of scientific evidence. However, it is important to note that there are many more strains that may be effective but have yet to be studied.
Health Benefits of Probiotics
Probiotics differ in their effects on human health. Most probiotics are from the genera Bifidobacterium or Lactobacillus. Various species of Bifidobacterium (B. adolescentis, B. animalis, B. bifidum, B. breve, and B. longum) and Lactobacillus (L.acidophilus, L. casei, L. fermentum, L. gasseri, L. johnsonii, L. paracasei, L. plantarum, and L. rhamnosus) provide general health benefits (Health Canada 2009). The dose of the probiotic taken, measured in "colony forming units", or CFUs, is important for effectiveness. A minimum dose of 1 × 109 (1 billion) CFUs per serving is thought to be needed to provide general health benefits (Health Canada 2009).
Probiotics exert health benefits by a number of mechanisms. Most probiotics inhibit the growth of pathogens and produce beneficial fermentation products such as short-chain fatty acids (Hill et al. 2014, Oelschlaeger 2010). Many probiotic species produce vitamins and useful enzymes and help to maintain gut health. A few probiotic strains have immune, neurological, or other body system effects, and some probiotic strains are considered drugs because they have been shown to prevent or treat disease (McFarland, Evans, and Goldstein 2018).
Digestive Health
Constipation
One common gastrointestinal condition is constipation. Those suffering from constipation often complain of infrequent, hard stools that are difficult to pass. A lack of dietary fiber, medical conditions, and certain medications are common causes of constipation.
One of the main functions of the colon is the removal of water. This is important for the prevention of diarrhea. However, the longer material remains in the colon, the more water is removed. If the time spent in the colon is lengthy, the result may be constipation. Transit time is the time it takes for the contents of the gastrointestinal tract to move through the body. Most of this time is spent in the colon. When transit time is slow, lasting many days, the result is hard stools that are difficult to pass. A stool form or consistency rating is a useful indicator of transit time (hard stools = slow transit; soft stools = normal transit; liquid stools = fast transit).
Probiotics are effective in speeding up transit time in adults and are most effective in people with constipation (Dimidi et al. 2014; Miller, Zimmermann, and Ouwehand 2016; Zhang et al. 2020). Stool consistency responds to changes in transit time and may improve with certain probiotics. A systematic review supports that probiotics, specifically multistrain probiotics, significantly improved stool consistency (stools became softer) and stool frequency (Zhang et al. 2020). In contrast, a previous review, including different studies, provided some evidence that single-strain probiotics may be more effective than the multistrain probiotics (Dimidi et al. 2014). There is also conflicting evidence regarding the effectiveness of Bifidobacterium lactis (B. lactis) strains on constipation outcomes (Zhang et al. 2020; Dimidi et al. 2014), and thus more research is needed.
Diarrhea
Diarrhea is commonly defined as frequent loose or watery stools and is often caused by pathogens. Potential probiotics have been evaluated in many studies to determine if they are effective in preventing various types of diarrhea in adults, including antibiotic-associated diarrhea and chemotherapy-induced diarrhea. Clostridium difficile diarrhea is the most common antibiotic-associated diarrhea.
Evidence from a systematic review and meta-analysis supports that probiotics reduce the incidence of Clostridium difficile-associated diarrhea and other antibiotic-associated diarrheas, with Lactobacillus casei being the most effective (Ma et al. 2020). Also, duration of diarrhea and the time until onset of diarrhea improved with various probiotics (Ma et al. 2020). A previous review of trials using Lactobacillus spp., Saccharomyces spp., and combinations of probiotics also showed reduced risk for diarrhea (Lau and Chamberlain 2016). However, a meta-analysis, which separated participants by age, found that probiotic administration did not reduce the risk of antibiotic-associated diarrhea in adults over 65 years of age (Jafarnejad et al. 2016).
Meta-analyses have also been carried out on specific strains of probiotics and their efficacy in antibiotic-associated diarrhea. Lactobacillus rhamnosus GG, Lactobacillus casei, Bacillus clausii, Saccharomyces boulardii, Lactobacillus acidophilus, and Multi-genera II are more effective than placebo in helping to prevent antibiotic-associated diarrhea (Cai et al. 2018). Lactobacillus rhamnosus GG is effective in decreasing risk of antibiotic-associated diarrhea in children, but in adults, Lactobacillus rhamnosus GG effective was only in those receiving antibiotics for H. pylori eradication (see section below) (Szajewska and Kolodziej 2015a). Saccharomyces boulardii is effective in decreasing risk of antibiotic-associated diarrhea in adults, but not specifically Clostridium difficile-associated diarrhea (Szajewska and Kolodziej 2015b).
A meta-analysis has shown that probiotics decrease radiotherapy-induced diarrhea but not chemotherapy-induced diarrhea in patients with abdominal and pelvic cancer (Wang et al. 2016). However, a newer meta-analysis showed that the application of probiotics before or during chemotherapy effectively prevented the occurrence of chemotherapy-induced diarrhea among cancer patients (Lu et al. 2019).
Irritable Bowel Syndrome (IBS)
Irritable Bowel Syndrome (IBS) is a condition of the intestinal tract that results in abdominal pain and/or discomfort with altered bowel habits (diarrhea and/or constipation).
A systematic review using the medical diagnostic criteria of IBS (Rome III) showed that probiotics improved overall symptom scores and quality of life in individuals with IBS (Zhang, Li, et al. 2016). Probiotic doses of <1010 CFU and single strain probiotics may be more effective than multistrain formulations for symptom relief. Specifically, Lactobacillus acidophilus-SDC, Lactobacillus plantarum 299v, Bacillus coagulans, and Bifidobacterium bifidum MIMBb75 improved overall symptom scores in patients with IBS, and Bifidobacterium bifidum MIMBb7 improved quality of life. A more recent systematic review and meta-analysis of studies using Rome I, II, or III to diagnose individuals with IBS found that probiotics significantly improved overall IBS symptoms (Asha and Khalil 2020). However, the meta-analysis showed no differences in abdominal pain scores with probiotics in general compared to placebos, but abdominal pain scores were reduced with probiotics containing Lactobacillus spp.
H. pylori Infection and Peptic Ulcer Disease
Infection with Helicobacter pylori (H. pylori), a bacterial pathogen, causes gastritis (inflammation of the stomach lining) and, if left untreated, may lead to gastric ulcers and cancer. The current recommended treatment for H. pylori is a combination of antibiotics with a proton-pump inhibitor (stomach acid-reducing medication) (Li et al. 2015). However, the treatment may cause nausea, vomiting, and diarrhea.
Various probiotic mixtures have been evaluated for their efficacy for improving the treatment (eradication rates) of H. pylori infection and preventing side effects from treatment (Lu, Yu, et al. 2016; McFarland et al. 2016; Lv et al. 2015; Zhang et al. 2015; Gong, Li, and Sun 2015). In a meta-analysis examining 40 controlled trials, probiotics improved the eradication rate and decreased the side effects when added to the antibiotic treatments designed to eradicate H. pylori, especially when used before and during the eradication treatment and for more than 2 weeks (Shi et al. 2019). Compared with a control group, Lactobacillus spp., Saccharomyces spp., and multiple strains demonstrated significant improvements in eradication rates. Other strains may be effective, but more research is needed.
Diverticular Disease
Diverticular disease is a common gastrointestinal disease, especially in older adults. Many older adults have diverticulosis (colonic diverticula, i.e., outpouching of the colon) but are without symptoms. Others develop symptoms such as abdominal pain, discomfort, and changes in bowel habit. A minority of individuals with diverticulosis develop diverticulitis, an acute inflammation of the diverticula.
There have been very few quality studies evaluating the effect of probiotics on diverticular disease (Lahner et al. 2016). Probiotics may potentially be beneficial in the management of symptoms of diverticular disease; however, much more research is needed before recommendations can be made.
Metabolism
Body Weight
Although probiotics are most often associated with gastrointestinal health, they may also have systemic effects, including an influence on body weight. In a recent review with meta-analysis multistrain and single-strain probiotics were reduced body weight, body mass index, waist circumference, fat mass, and fat percentage (Wang et al. 2019). The effects were stronger with high dose and single-strain probiotics. Similar findings were confirmed by an second review (Koutnikova et al. 2019). Currently, no specific recommendations can be made regarding the best strain combination and dose for body weight reduction.
Type 2 Diabetes
The goal of successful management of type 2 diabetes is to achieve near-normal fasting blood glucose and hemoglobin A1c, a blood test used to estimate average blood glucose levels over a three-month period. In three reviews of studies evaluating probiotics in participants with type 2 diabetes, fasting blood glucose was lower with probiotic supplementation compared to the placebo (Samah et al. 2016; Zhang, Wu, and Fei 2016; Li et al. 2016). The reviews differed on their findings with respect to probiotics and A1c, with one study showing a lowering and two showing no effect. However, the studies included were only four to eight weeks in length—too short to expect changes in A1c. In a more recent review of studies and meta-analysis evaluating the effect of oral intake of probiotics on variables related to obesity, diabetes, and nonalcoholic fatty liver disease, it was found that probiotics reduced fasting glucose, glycated hemoglobin, and insulin (Koutnikova et al. 2019). These improvements were observed with mixtures containing Bifidobacterium breve, Bifidobacterium longum, Streptococcus salivarius subsp. thermophilus, Lactobacillus acidophilus, Lactobacillus casei, and Lactobacillus delbrueckii.
Cholesterol
Elevated serum total cholesterol and LDL (low-density lipoprotein) cholesterol are risk factors for cardiovascular disease. Consumption of probiotics has been found to significantly decrease total and LDL cholesterol (Cho and Kim 2015). Probiotics had no effect on HDL (high-density lipoprotein) cholesterol or triglycerides. Lactobacillus acidophilus alone and in combination with L. lactis and L. plantarum showed independent beneficial effects. As with other cholesterol trials, participants with the highest cholesterol levels showed the greatest benefits. A more recent review of studies concluded that administration of probiotics resulted in significant reductions of total cholesterol, LDL cholesterol, and triglycerides and increased HDL cholesterol (Tenorio-Jiménez et al. 2020). However, some of the studies evaluated used the probiotics in combination with other compounds, which makes it more difficult to determine the specific effects of the probiotics.
Infection
Urinary Tract Infections
The effects of a variety of strains, formulations, and doses of probiotics on the prevention of urinary tract infections in healthy individuals have been evaluated (Schwenger, Tejani, and Loewen 2015). No benefit was found, but most studies were small and of poor quality. More research is needed to determine if there is a relationship between probiotic consumption and urinary tract infections.
Respiratory Infections
Upper respiratory tract infections (URTI), such as the common cold, are often due to viruses. For a number of years, meta-analysis evidence has supported the role of oral intake of probiotics and potential probiotics in decreasing the incidence of acute URTI, the duration of URTI, and related antibiotic use (Hao, Dong, and Wu 2015). However, confirmatory research studies of improved quality are needed. A review of studies and meta-analysis using several probiotic formulations, including those containing Lactobacillus and Bifidobacterium spp., found that infants and children who received probiotics to prevent acute illnesses had a lower risk of being prescribed antibiotics, compared to those who received a placebo (King et al. 2018).
Periodontal Disease
Probiotics may be beneficial in the prevention and treatment of dental caries and periodontal disease. However, a recent systematic review showed that four studies found benefits of using probiotics as an adjunctive therapy in chronic periodontitis, whereas three studies found no benefits compared to placebo (Ikram et al. 2018). Probiotics, Bifidobacterium spp. in particular, have been shown to reduce populations of Streptococcus mutans, a bacteria linked to dental caries, and also may help manage gingivitis and periodontitis (Gruner, Paris, and Schwendicke 2016).
Probiotic Purchasing Tips
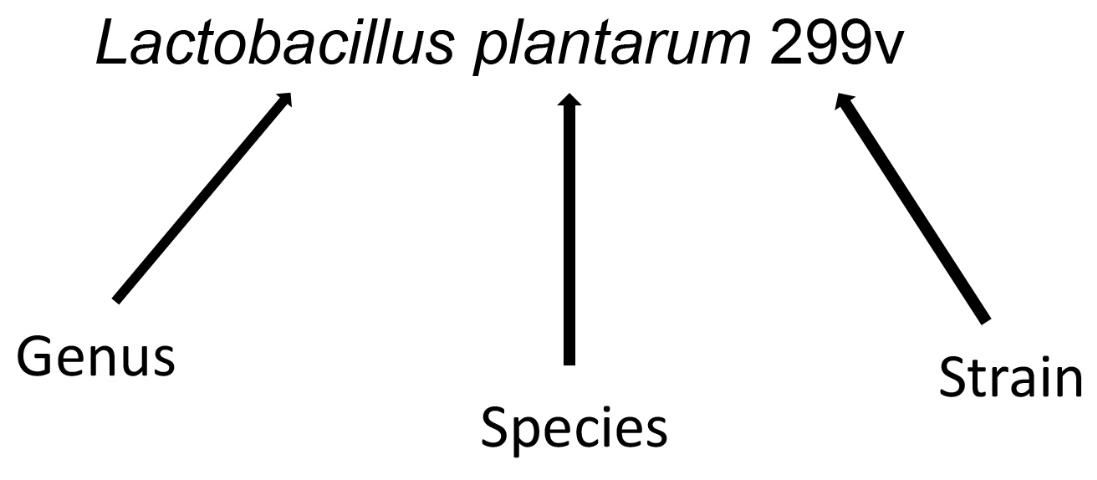
Although more research is needed to determine the health effects of many potential probiotics, it is wise to select a probiotic that has been well studied and has known health benefits. When choosing a probiotic, ensure that the probiotic is described by genus, species, and strain on the label (see Figure 1 below). When making a purchasing decision, look for information on the genus and species and the health benefit you are seeking. If the health effect is strain-specific, look for the strain (named by genus, species, and strain number) information. The label should clearly indicate the number of viable cells (CFUs), which should exceed, at a minimum, 1 × 109 (1 billion). Follow the manufacturer's recommendation for storage because some probiotics require refrigeration, while many others can be stored at room temperature.
References
Asha, M. Z., and S. F. H. Khalil. 2020. "Efficacy and Safety of Probiotics, Prebiotics and Synbiotics in the Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta- Analysis." Sultan Qaboos University Medical Journal 20 (1): e13–e24. https://doi.org/10.18295/squmj.2020.20.01.003
Cho, Y. A., and J. Kim. 2015. "Effect of Probiotics on Blood Lipid Concentrations: A Meta-Analysis of Randomized Controlled Trials." Medicine (Baltimore) 94 (43): e1714. https://doi.org/10.1097/md.0000000000001714
Dimidi, E., S. Christodoulides, K. C. Fragko, S. M. Scott, and K. Whelan. 2014. "The Effect of Probiotics On Func- tional Constipation In Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials." American Journal of Clinical Nutrition 100 (4): 1075–1084. https://doi.org/10.3945/ajcn.114.089151
Food and Agricultural Organization of the United Nations and World Health Organization. 2002. Guidelines for the Evaluation of Probiotics in Food. Food and Agricultural Organization of the United Nations. https://isappscience.org/wp-content/uploads/2019/04/probiotic_guidelines.pdf. Accessed August 5, 2022.
Goldenberg, J. Z., S. S. Ma, J. D. Saxton, M. R. Martzen, P. O. Vandvik, K. Thorlund, G. H. Guyatt, and B. C. Johnston. 2013. "Probiotics for the Prevention of Clostridium difficile- Associated Diarrhea in Adults and Children." The Cochrane Database of Systematic Review (5):Cd006095. https://doi. org/10.1002/14651858.CD006095.pub4
Gong, Y., Y. Li, and Q. Sun. 2015. "Probiotics Improve Efficacy and Tolerability of Triple Therapy to Eradicate Helicobacter Pylori: A Meta-Analysis of Randomized Controlled Trials." The International Journal of Clinical and Experimental Medicine 8 (4): 6530–43. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4483842/
Gruner, D., S. Paris, and F. Schwendicke. 2016. "Probiotics for Managing Caries and Periodontitis: Systematic Review and Meta-Analysis." Journal of Dentistry 48:16–25. https://doi.org/10.1016/j.jdent.2016.03.002
Hao, Q., B. R. Dong, and T. Wu. 2015. "Probiotics for Preventing Acute Upper Respiratory Tract Infections." The Cochrane Database of Systematic Review 2:Cd006895. https://doi.org/10.1002/14651858.CD006895.pub3
Health Canada. 2009. "Accepted Claims about the Nature of Probiotic Microorganisms in Food." Accessed August 5, 2022. https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/accepted-claims-about-nature-probiotic-microorganisms-food.html
Hill, C., F. Guarner, G. Reid, G. R. Gibson, D. J. Merenstein, B. Pot, L. Morelli, R. B. Canani, H. J. Flint, S. Salminen, P. C. Calder, and M. E. Sanders. 2014. "The International Scientific Association for Probiotics and Prebiotics Consensus Statement on The Scope and Appropriate Use of the Term Probiotic." Nature Reviews Gastroenterology & Hepatology 11 (8): 506–14. https://doi.org/10.1038/nrgastro.2014.66
Ikram, S., N. Hassan, M. A. Raffat, S. Mirza, and Z. Akram. 2018. "Systematic Review and Meta-Analysis of Double-Blind, Placebo-Controlled, Randomized Clinical Trials Using Probiotics in Chronic Periodontitis." Journal of Investigative and Clinical Dentistry 9 (3): e12338. https://doi.org/10.1111/jicd.12338
Jafarnejad, S., S. Shab-Bidar, J. R. Speakman, K. Parastui, M. Daneshi-Maskooni, and K. Djafarian. 2016. "Probiotics Reduce the Risk of Antibiotic-Associated Diarrhea in Adults (18–64 Years) but Not the Elderly (>65 Years): A Meta-Analysis." Nutrition in Clinical Practice 31 (4): 502–13. https://doi.org/10.1177/0884533616639399
King, S., D. Tancredi, I. Lenoir-Wijnkoop, K. Gould, H. Vann, G. Connors, M. E. Sanders, J. A. Linder, A. L. Shane, and D. Merenstein. 2018. "Does Probiotic Consumption Reduce Antibiotic Utilization for Common Acute Infections? A Systematic Review and Meta-Analysis." European Journal of Public Health 29 (3): 494–499. https://doi.org/10.1093/eurpub/cky185
Koutnikova, H., B. Genser, M. Monteiro-Sepulveda, J. M. Faurie, S. Rizkalla, J. Schrezenmeir, and K. Clément. 2019. "Impact of Bacterial Probiotics on Obesity, Diabetes and Non-Alcoholic Fatty Liver Disease Related Variables: A Systematic Review and Meta-Analysis of Randomised Controlled Trials." BMJ 9 (3): e017995. https://doi.org/10.1136/bmjopen-2017-017995
Lahner, E., C. Bellisario, C. Hassan, A. Zullo, G. Esposito, and B. Annibale. 2016. "Probiotics in the Treatment of Diverticular Disease. A Systematic Review." Journal of Gastrointestinal and Liver Diseases 25 (1): 79–86. https://doi.org/10.15403/jgld.2014.1121.251.srw
Lau, C. S., and R. S. Chamberlain. 2016. "Probiotics Are Effective at Preventing Clostridium difficile-Associated Diarrhea: A Systematic Review and Meta-Analysis." Inter- national Journal of General Medicine 9:27–37. https://doi.org/10.2147/ijgm.s98280
Li, B. Z., D. E. Threapleton, J. Y. Wang, J. M. Xu, J. Q. Yuan, C. Zhang, P. Li, Q. L. Ye, B. Guo, C. Mao, and D. Q. Ye. 2015. "Comparative Effectiveness and Tolerance of Treatments For Helicobacter Pylori: Systematic Review And Network Meta-Analysis." BMJ 351:h4052. https://doi. org/10.1136/bmj.h4052
Li, C., X. Li, H. Han, H. Cui, M. Peng, G. Wang, and Z. Wang. 2016. "Effect of Probiotics on Metabolic Profiles in Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized, Controlled Trials." Medicine (Baltimore) 95 (26): e4088. https://doi.org/10.1097/md.0000000000004088
Lu, D., J. Yan, F. Liu, P. Ding, B. Chen, Y. Lu, and Z. Sun. 2019. "Probiotics in Preventing and Treating Chemotherapy-Induced Diarrhea: A Meta-Analysis." Asia Pacific Clinical Nutrition Society 28 (4): 701–710. https://doi.org/10.6133/apjcn.201912_28(4).0005
Lu, M., S. Yu, J. Deng, Q. Yan, C. Yang, G. Xia, and X. Zhou. 2016. "Efficacy of Probiotic Supplementation Therapy for Helicobacter pylori Eradication: A Meta-Analysis of Ran- domized Controlled Trials." PLoS One 11 (10): e0163743. https://doi.org/10.1371/journal.pone.0163743
Lv, Z., B. Wang, X. Zhou, F. Wang, Y. Xie, H. Zheng, and N. Lv. 2015. "Efficacy and Safety of Probiotics as Adjuvant Agents for Helicobacter pylori Infection: A Meta-Analysis." Experimental and Therapeutic Medicine 9 (3): 707–716. https://doi.org/10.3892/etm.2015.2174
Ma, Y., J. Y. Yang, X. Peng, K. Y. Xiao, Q. X., and C. Wang. 2020. "Which Probiotic Has the Best Effect on Preventing Clostridium difficile-Associated Diarrhea? A Systematic Review and Network Meta-Analysis." Journal of Digestive Diseases 21 (2): 69–80. https://doi.org/10.1111/1751-2980.12839
McFarland, L. V. 2016. "An Observation on Inappropriate Probiotic Subgroup Classifications in the Meta-Analysis by Lau and Chamberlain." International Journal of General Medicine 9:333–336. https://doi.org/10.2147/ijgm.s119970
McFarland, L. V., C. T. Evans, and E. J. Goldstein. 2018. "Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis." Frontiers in Medicine 5 (124). https://doi.org/10.3389/fmed.2018.00124
McFarland, L. V., Y. Huang, L. Wang, and P. Malfertheiner. 2016. "Systematic Review and Meta-Analysis: Multi-Strain Probiotics as Adjunct Therapy for Helicobacter pylori Eradication and Prevention of Adverse Events." United European Gastroenterology Journal 4 (4): 546–61. https://doi.org/10.1177/2050640615617358
McFarland, L. V., P. Malfertheiner, Y. Huang, and L. Wang. 2015. "Meta-Analysis of Single Strain Probiotics for the Eradication of Helicobacter pylori and Prevention of Adverse Events." World Journal of Meta-Analysis 3 (2): 97–117. https://doi.org/10.13105/wjma.v3.i2.97
Miller, L. E., A. K. Zimmermann, and A. C. Ouwehand. 2016. "Contemporary Meta-Analysis of Short-Term Probiotic Consumption on Gastrointestinal Transit." World Journal of Gastroenterology 22 (21): 5122–31. https://doi.org/10.3748/wjg.v22.i21.5122
Oelschlaeger, T. A. 2010. "Mechanisms of Probiotic Actions—A Review." International Journal of Medical Microbiology i 300 (1): 57–62. https://doi.org/10.1016/j.ijmm.2009.08.005
Pattani, R., V. A. Palda, S. W. Hwang, and P. S. Shah. 2013. "Probiotics for the Prevention of Antibiotic-Associated Diarrhea and Clostridium difficile Infection among Hospitalized Patients: Systematic Review and Meta-Analysis." Open Medicine 7 (2): e56–67. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3863752/
Samah, S., K. Ramasamy, S. M. Lim, and C. F. Neoh. 2016. "Probiotics for the Management of Type 2 Diabetes Mel- litus: A Systematic Review and Meta-Analysis." Diabetes Research and Clinical Practice 118:172–182. https://doi.org/10.1016/j.diabres.2016.06.014
Sanchez, B., S. Delgado, A. Blanco-Miguez, A. Lourenco, M. Gueimonde, and A. Margolles. 2016. "Probiotics, Gut Microbiota and Their Influence on Host Health and Disease." Molecular Nutrition and Food Research 61 (1): 1600240. https://doi.org/10.1002/mnfr.201600240
Schwenger, E. M., A. M. Tejani, and P. S. Loewen. 2015. "Probiotics for Preventing Urinary Tract Infections in Adults and Children." Cochrane Database of Systematic Review (12):Cd008772. https://doi.org/10.1002/14651858.CD008772.pub2
Shi, X., J. Zhang, L. Mo, J. Shi, M. Qin, and X. Huang. 2019. "Efficacy and Safety of Probiotics in Eradicating Helicobacter pylori: A Network Meta-Analysis." Medicine 98 (15): e15180–e15180. https://doi.org/10.1097/MD.0000000000015180
Szajewska, H., and M. Kolodziej. 2015a. "Systematic Review with Meta-Analysis: Lactobacillus rhamnosus GG in the Prevention of Antibiotic-Associated Diarrhoea in Children and Adults." Alimentary Pharmacology & Therapeutics 42 (10): 1149–57. https://doi.org/10.1111/apt.13404
Szajewska, H., and M. Kolodziej. 2015b. "Systematic Review with Meta-Analysis: Saccharomyces boulardii in the Prevention of Antibiotic-Associated Diarrhoea." Alimentary Pharmacology & Therapeutics 42 (7): 793–801. https://doi.org/10.1111/apt.13344
Tenorio-Jiménez, C., M. J. Martínez-Ramírez, A. G., and C. Gómez-Llorente. 2020. "Effects of Probiotics on Metabolic Syndrome: A Systematic Review of Randomized Clinical Trials." Nutrients 12 (1): 124. https://doi.org/10.3390/nu12010124
Wang, Y. H., N. Yao, K. K. Wei, L. Jiang, S. Hanif, Z. X. Wang, and C. X. Pei. 2016. "The Efficacy and Safety of Probiotics for Prevention of Chemoradiotherapy-Induced Diarrhea in People with Abdominal and Pelvic Cancer: A Systematic Review and Meta-Analysis." European Journal of Clinical Pharmacology 70 (11): 1246–1253. https://doi.org/10.1038/ejcn.2016.102
Wang, Z. B., S. S. Xin, L. N. Ding, W. Y. Ding, Y. L. Hou, C. Q Liu, and X. D. Zhang. 2019. “The Potential Role of Probiotics in Controlling Overweight/Obesity and Associated Metabolic Parameters in Adults: A Systematic Review and Meta-Analysis.” Evidence-Based Complementary and Alternative Medicine 2019:3862971. https://doi.org/10.1155/2019/3862971
C. Q Liu, and X. D. Zhang. 2019. "The Potential Role of Probiotics in Controlling Overweight/Obesity and Associ- ated Metabolic Parameters in Adults: A Systematic Review and Meta-Analysis." Evidence-Based Complementary and Alternative Medicine 2019:3862971. https://doi.org/10.1155/2019/3862971
Zhang, C., J. Jiang, F. Tian, J. Zhao, H. Zhang, Q. Zhai, and W. Chen. 2020. "Meta-analysis of Randomized Controlled Trials of The Effects of Probiotics on Functional Constipation in Adults." Clinical Nutrition 39 (10): 2960–69. https://doi.org/10.1016/j.clnu.2020.01.005
Zhang, M. M., W. Qian, Y. Y. Qin, J. He, and Y. H. Zhou. 2015. "Probiotics in Helicobacter pylori Eradication Therapy: A Systematic Review and Meta-Analysis." World Journal of Gastroenterology 21 (14): 4345–57. https://doi.org/10.3748/wjg.v21.i14.4345
Zhang, Q., Y. Wu, and X. Fei. 2016. "Effect of Probiotics on Glucose Metabolism in Patients with Type 2 Diabetes Mel- litus: A Meta-Analysis of Randomized Controlled Trials." Medicina (Kaunas) 52 (1): 28–34. https://doi.org/10.1016/j.medici.2015.11.008
Zhang, Y., L. Li, C. Guo, D. Mu, B. Feng, X. Zuo, and Y. Li. 2016. "Effects of Probiotic Type, Dose and Treatment Duration on Irritable Bowel Syndrome Diagnosed by Rome III Criteria: A Meta-Analysis." BMC Gastroenterology 16 (1): 62. https://doi.org/10.1186/s12876-016-0470-z

