Introduction
If you have a strong allergic reaction to sulfites you may already know some ways to avoid the substances. However, even with today's technology, food recalls due to undeclared sulfite (as a food ingredient) continue to occur in the United States. This publication will examine what sulfites are, sulfite sensitivity, safety issues related to sulfiting agents, and recommendations for sulfite-sensitive individuals.
What are sulfites? Sulfites are inorganic salts that have antioxidant and preservative properties. Many compounds capable of producing sulfite, called sulfiting agents, have been used as food additives since antiquity to help prevent enzymatic and nonenzymatic browning; control growth of microorganisms; act as bleaching agents, antioxidants, or reducing agents; and carry out various other technical functions (Sapers 1993; Taylor et al. 1986). Examples of sulfiting agents include sulfur dioxide, sodium sulfate, sodium and potassium bisulfites, and metabisulfites. Specifically, sulfites are used on fruits and vegetable to prevent unpleasant browning, on shrimp and lobster to prevent melanosis, or "black spot", in wines to discourage bacterial growth, in dough as a conditioner, and to bleach certain food starches and cherries. In addition, sulfites are used in pharmaceuticals to maintain the stability and potency of some medications (Knodel 1997; Papazian 1996).
Sulfite treatment levels in foods vary widely by application. Residual levels do not usually exceed several hundred parts per million (ppm) but may approach 1,000 ppm in certain fruit and vegetable products (Sapers 1993; Taylor et al. 1986).
In fresh fruits and vegetables, sulfites prevent an enzyme called polyphenol oxidase (PPO) from working properly in order to prevent brown pigment formation (Sapers 1993; Sayavedra-Soto and Montgomery 1986). Sulfites are thought to inhibit browning by acting as a reducing agent that combines with the ortho-quinones and converts them back to colorless diphenols. This prevents the nonenzymatic condensation of o-quinones to complex brown polymers as seen in Figure 1. (Weaver 1974).
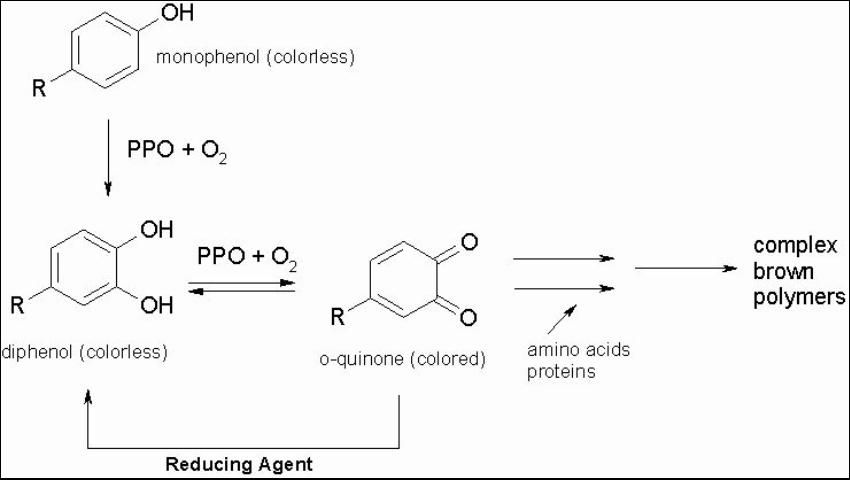
Credit: Adapted from Walker (1977)
Sulfites occur naturally in some foods and beverages as a result of fermentation, such as in beer and wine. As a food additive, sulfites have been used since 1664 and have been approved for use in the United States since the 1800s (Lester 1995). With such a history of use, sulfites have been generally regarded as safe (GRAS) by the FDA, however it is suspected that a small percentage of the population is sensitive to sulfites. This sensitivity can cause a wide range of reactions ranging from mild to severe.
Sulfite Sensitivity
Sensitivity to sulfites can develop at any time during a person's lifespan, with some initial reactions not showing up until a person has reached their forties or fifties. The manifestations of sulfite sensitivity include a large array of dermatological, pulmonary, gastrointestinal, and cardiovascular symptoms. Asthmatics that are steroid-dependent or have a great degree of airway hyperreactivity may be at an increased risk of having a reaction to a sulfite containing food (Lester 1995). Varying degrees of bronchospasm, angiodema, urticaria, nausea, abdominal cramping, and diarrhea are commonly reported (Knodel 1997). Adverse reactions to sulfites in nonasthmatics are extremely rare.
Although literature lists a range of figures as to what percent of the population is affected, the Food and Drug Administration (FDA) estimates that one out of a hundred people is sulfite-sensitive, and of that group 5% have asthma. Another source states that 5% of asthmatics are sulfite sensitive, compared to only 1% of the nonasthmatic population (Knodel 1997), while another source estimates that up to 500,000 (or less than .05% of the population) sulfite-sensitive individuals live in the United States (Lester 1995).
Symptoms of sulfite intolerance can occur within 5 minutes following parenteral exposure and within 15–30 minutes following oral exposure. Sensitive individuals vary in their degree of intolerance towards sulfites, with each having a specific threshold of exposure needed to elicit a reaction (Knodel 1997). While the majority of reactions are mild, severe nonspecific signs and symptoms do occur on occasion. Although the precise mechanisms of the sensitivity responses to sulfites have not been completely elucidated, three have been implicated: inhalation of sulfur dioxide (SO2) generated in the stomach proceeding ingestion of sulfite-containing foods or beverages; deficiency in a mitochondrial enzyme; or an IgE-mediated immune response (Lester 1995).
Other Safety Concerns and Regulations
Sulfiting agents are not teratogenic, mutagenic, or carcinogenic in laboratory animals, however there is a fraction of the public that is sulfite sensitive and susceptible to a wide range of effects due to acute allergic reactions (FDA 1988). The FDA regulates the use of sulfites in drugs and food, while the United States Department of Agriculture (USDA) regulates the use of sulfites in meat and poultry. The Bureau of Alcohol, Tobacco and Firearms (BATF) regulates the use of sulfites in alcoholic beverages and the use of sulfur dioxide as a fungicide on grapes comes under the authority of the Environmental Protection Agency (EPA).
Sulfites have such a long history of use that in 1958, when the Federal Food, Drug, and Cosmetic Act was amended to regulate preservatives and other food additives, the FDA classified sulfites as generally recognized as safe (GRAS) (Papazian 1996). However, in response to a 1982 FDA proposal to affirm the GRAS status of sulfating agents, the FDA began to receive reports of adverse health reactions to sulfites (Papazian 1996; Sapers 1993). The FDA contracted the Federation of American Societies for Experimental Biology (FASEB) to examine the link between sulfites and the reported health claims.
The FASEB submitted its final report to the FDA in 1985, concluding that sulfites are safe for most people, but could pose a hazard of unpredictable severity to asthmatics and others who are sensitive to them. In 1986, the FDA took the following regulatory actions based on the FASEB report:
- Prohibited the use of sulfites to maintain color and crispness of fresh fruits and vegetables, such as in salad bars or fresh produce in supermarkets.
- Required companies to list sulfites or chemical components that give rise to sulfites with at least 10 parts per million (ppm) or higher, and any sulfating agents that had a technical or functional effect in the food regardless of the amount present. (The equivalent "ink concentration" would be 40 drops in a 55-gallon barrel of water)
(Papazian 1996)
In 1987, the FDA proposed to revoke the GRAS status of sulfiting agents on fresh potatoes intended to be cooked and served unpackaged and unlabeled to consumers and issued a final ruling to this effect in 1990. However, the rule was held null and void in 1990 after a protracted court battle in which the potato industry prevailed on procedural grounds (Papazian 1996). Also in 1987 FDA regulations went into effect that required manufacturers of drugs to include a warning label on all prescription drugs to which sulfites have been added.
In 1988, the FDA proposed new rules that would require the presence of sulfites in standardized foods be declared on the label when the sulfating agents have a functional effect or are present at a detectable level, defined as 10 ppm or more (FDA 1988a, FDA 2022). Additional rules affirmed the GRAS status of sulfating agents in certain specified foods at specified maximum residual levels, provided that the presence of sulfite is declared on the label of packaged products or on bulk containers "plainly in view" of the purchaser or is indicated by a counter sign, card, or another device bearing information that the product has been treated with sulfites (Sapers 1993).
The USDA prohibits the use of sulfites on meat because they may give an appearance of "false freshness" by restoring the red color to raw meat. However, ingredients treated with sulfites may be added to meat in preparation of certain processed foods, beef stew for example. The USDAs Food Safety and Inspection Service (FSIS) adopted a labeling policy for processed meat products consistent with the FDAs regulation (10 ppm or higher require labeling of sulfites). Sulfites must also be declared if they make up one part of a multi-component dinner that contains 10 ppm or more of sulfites, even if the entire dinner contains a lower level than that.
Current Regulation Status of Sulfites
Currently, sulfiting agents are not considered GRAS for use in meats, foods recognized as a major source of vitamin B-1 (sulfites have been found to destroy thiamin ), or "fruits or vegetables intended to be served raw to consumers or to be presented to consumers as fresh" (FDA 1988b).
The FDA regulations do not require the managers of food service establishments to disclose to consumers if sulfites were used in the food preparation (Papazian 1996). Consumers should therefore be careful and not expect the wait staff at restaurants to know this information, as erroneous information may be given. Consumers with sulfite sensitivity must become savvy at deducing whether a particular restaurant food might contain sulfites based on whether a similar packaged good is labeled as containing sulfating agents. It is also important that the sulfite-sensitive individual be aware of the large array of cooked and processed foods that still contain sulfites such as:
- Baked goods.
- Condiments, dried and glacéed fruit, jam, and molasses.
- Gravy, dehydrated or pre-cut or peeled potatoes, shrimp, and soup mix.
- Beverages such as beer, wine, hard cider, fruit and vegetable juice, and tea (Papazian 1996).
*A list of foods that may contain sulfites may be found in the appendix.
Although the majority of sulfite-sensitive reactions are reported in conjunction with ingestion of food, a number of reactions have been attributed to drug products containing sulfites. Therefore, it is imperative that doctors be consulted regarding the sulfite content of drugs to be dispensed or administered to a sulfite-sensitive individual (Knodel 1997).
Recommendations for Sulfite-Sensitive Individuals
The following are measures those with sensitivity to sulfites should take when buying unlabeled foods at a deli, supermarket, or food service establishment:
- If the food is being sold loose or by portion, ask the store manager or waiter to check the ingredient list on the products original bulk size packaging.
- Avoid processed foods that contain sulfites, such as dried fruits, canned vegetables, maraschino cherries, and guacamole.
- When ordering a potato, opt for a baked potato over any kind that involves peeling of the vegetable during preparation.
If you have asthma, don't go out to eat without your inhaler! If you've experienced a reaction to sulfites in the past, carry an antihistamine with you, and make sure you have self-injectable epinephrine in order to stabilize your condition until you can reach an emergency room (Knodel 1997; Papazian 1996).
Summary
This paper examines the definition of sulfites, sulfite sensitivity, safety issues related to sulfiting agents, and recommendations sulfite-sensitive individuals should take in order to protect themselves. Sulfites are inorganic salts that have antioxidant and preservative properties. Sulfites have been used as a food additive since 1664 and have been approved for use in the United States for more than a century. Due to their history of use, sulfites have been generally regarded as safe, however there is also a small percentage of the population that is suspected of being sensitive to sulfites. This sensitivity can cause a wide range of reactions ranging from mild to severe; therefore the proper precautions should be taken.
References
FDA. 1988a. Sulfiting agents in standardized foods: Labeling requirements. Food and Drug Admin., Fed Reg. 53: 51062–51084.
FDA. 1988b. Sulfiting agents: Affirmation of GRAS status. Food and Drug Admin., Fed Reg. 53: 51065–51084.
FDA. 2022. Sulfites in standardized food (21 CFR 130.9 Sulfite) https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=130.9, accessed 5-5-2022.
Knodel, L.C. 1997. Current Issues in Drug Toxicity; Potential health hazards of sulfites. Toxic Subst. Mech. 16(3): 309–311.
Lester, M.R. 1995. Sulfite sensitivity: Significance in human health. J. Am. Coll. Nutr. 14(3): 229–232.
Papazian, R. 1996. Sulfites: Safe for most, dangerous for some. FDA Consumer Magazine. 30(10).
Sapers, G.M. 1993. Browning of foods: control by sulfites, antioxidants, and other means. Food Technol. 47(10): 75–84.
Sayaverdra-Soto, L.A. and Montgomery, M.W. 1986. Inhibition of polyphenoloxidase by sulfite. J. Food Sci. 51: 1531–1536.
Taylor, S.L., Higley, N.A., and Bush, R.K. 1986. Sulfites in foods: Uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity, and hypersensitivity. Adv. Food Res. 30: 1–76.
Walker, J.R.L. 1977. Enzymatic browning in foods. Its chemistry and control. Food Technol. NZ, 12: 19–25.
Weaver, C.M. 1974. Factors influencing enzymatic browning of ripening bananas. Department of Foods and Nutrition. Oregon State University Master of Science Thesis.
Appendix: FDA GUIDE TO FOODS AND DRUGS WITH SULFITES
The following foods and drugs MAY contain sulfites, according to the Food and Drug Administration. Remember to check the product label.
Alcoholic Beverages: Beer, cocktail mixes, wine, and wine coolers
Baked Goods: Cookies, crackers, mixes with dried fruits or vegetables, pie crust, pizza crust, quiche crust, and flour tortillas
Beverage Bases: Dried citrus fruit beverage mixes
Condiments and Relishes: Horseradish, onion and pickle relishes, pickles, olives, salad dressing mixes, and wine vinegar
Confections and Frostings: Brown, raw, powdered or white sugar derived from sugar beets
Modified Dairy Products: Filled milk (a specially prepared skim milk in which vegetable oils, rather than animal fats, are added to increase its fat content)
Drugs: Antiemetics (taken to prevent nausea), cardiovascular drugs, antibiotics, tranquilizers, intravenous muscle relaxants, analgesics (painkillers), anesthetics, steroids and nebulized bronchodilator solutions (used for treatment of asthma)
Fish and Shellfish: Canned clams; fresh, frozen, canned, or dried shrimp; frozen lobster; scallops; dried cod
Fresh Fruit and Vegetables: Sulfite use banned (except for fresh potatoes)
Gelatins, Puddings, and Fillings: Fruit fillings, flavored and unflavored gelatin, and pectin jelling agents
Grain Products and Pastas: Cornstarch, modified food starch, spinach pasta, gravies, hominy, breadings, batters, noodle/rice mixes
Jams and Jellies: Jams and jellies
Nuts and Nut Products: Shredded coconut
Plant Protein Products: Canned, bottled, or frozen fruit juices (including lemon, lime, grape, and apple); dried fruit; canned, bottled, or frozen dietetic fruit or fruit juices; maraschino cherries and glazed fruit
Processed Vegetables: Vegetable juice, canned vegetables (including potatoes), pickled vegetables (including sauerkraut), dried vegetables, instant mashed potatoes, frozen potatoes, potato salad
Snack Foods: Dried fruit snacks, trail mixes, filled crackers
Soups and Soup Mixes: Canned seafood soups, dried soup mixes
Sweet Sauces, Toppings: Corn syrup, maple syrup, fruit toppings, and high-fructose syrups such as corn syrup and pancake syrup
Tea: Instant tea, liquid tea concentrates