Foliar application of nitrogen (N) and other nutrients essential for plant growth and development is an important practice for crop production. Most foliar nutrient N products contained the traditional N sources such as ammonium (NH4+), nitrate (NO3-) and/or urea (CH2N2O) before urea-triazone N was available on the market (Clapp 1993). The traditional N sources have a greater salt index and leaf-burn potential than urea-triazone N. Leaf burn and/or plant toxicity to foliage can be problematic for foliar application (Widders 1991). To avoid or minimize leaf burning, urea-triazone N fertilizers can be used instead of the traditional N sources (Widders 1991; Clapp 1993). However, limited information is available about urea-triazone-based fertilizers, and crop producers still have concerns about them. Growers have concerns about the safety of using triazone N fertilizers for commercial crop production because the fertilizers are still new to them. This article will provide basic information on urea-triazone-based fertilizer for UF/IFAS Extension faculty, crop consultants and advisors, growers, and students interested in commercial vegetable production.
Urea-triazone N is a patented, heterocyclic, organic N compound identified as S-tetrahydrotriazone by Hawkins (1985) and described by Landels et al. (1990). Urea-triazone N (trade name, N-SURE®) is produced by reacting urea, formaldehyde, and ammonia (Clapp 1991). Triazone gives foliar nitrogen fertilizers unique characteristics. For example, urea-triazone remains on the leaf surface in a liquid phase much longer than urea. Triazone itself is a low-volatile, safe, and stable source of slow-release N because it has a closed-ring structure with three carbon and three N atoms (Figure 1). The six atoms form a strong chemical bond and hence triazone releases N slowly, lowering burning potential and improving absorption efficiency. The leaf burn potential of urea-triazone is much less than ammonium, nitrate, and/or all urea-based fertilizers (Clapp 1993; Murray 2007) because urea-triazone is a slow-release N fertilizer. Triazone‐N is taken up into leaf tissue in similar quantities compared to urea, ammonium, and nitrate‐N (Widders 1991), as a solute, can also increase osmolality of the solution. Thus, if too much is added to an existing solution, triazone may have the possibility to exacerbate leaf burn, particularly when the temperature is greater than 90°F and the relative humidity is less than 30%. Under these conditions, the foliar nutrient solution added to the leaf can concentrate by evaporative loss of water. Additionally, leaf injury can be increased with N rate.
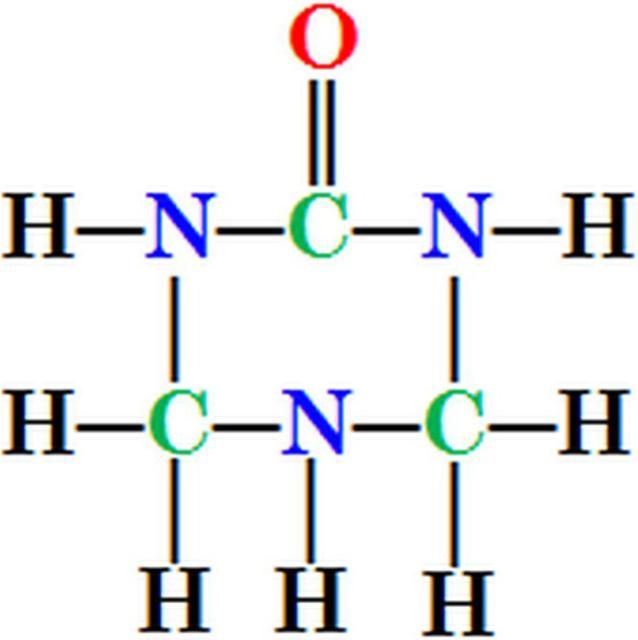
Credit: Guodong Liu, UF/IFAS
By weight, urea-triazone contains 28% total N including 7.8% urea N and 20.2% slowly available N from triazone. It has 3 pounds of N per gallon and 560 pounds N per ton. Its density is 10.72 lbs/gallon and volume value is 186.6 gallons/ton. Its pH is 9.5 and specific gravity, 1.286 (Clapp 1993). This liquid fertilizer may also be applied as a band or a sidedress, or it may be injected through drip, sprinkler, or center pivot irrigation systems. It is compatible with different phosphate and potash fertilizers and with most micronutrient fertilizers as well as many crop protection chemicals. However, special care should be taken when it is applied with fertilizers containing ammonium and/or ferrous iron because of its high pH. At such high pH, ammonium N is subject to ammonium volatilization; ferrous iron becomes unavailable to plants due to oxidation. Avoid mixing urea-triazone with fertilizers containing ammonium N and/or ferrous iron [Fe(II)].
In summary, urea-triazone is a slow-release N fertilizer containing 28% total N suitable for both foliar and soil application for commercial vegetable and fruit crops. This nitrogen fertilizer is different from most of other controlled release fertilizers. It is a stable solution and an effective N source for fertigation. It has low plant toxicity/burning potential and high compatibility with many P and K fertilizers but is not compatible with ammonium, and ferrous iron.
References
Clapp, J.P., Jr. 1993. "Foliar application of liquid urea-triazone-based nitrogen fertilizers and crop safety." HortTechnology 3(4): 442–444. https://journals.ashs.org/horttech/view/journals/horttech/3/4/article-p442.xml. Accessed on February 4, 2023.
Clapp, J.P., Jr. 1991. "Properties and uses of liquid urea-triazone-based nitrogen fertilizers." Fertilizer Research 28: 229-232. https://doi.org/10.1007/BF01049755. Accessed on January 15, 2023.
Hawkins, E.F. 1985. Triazone fertilizer and method of making. US Patent 4,599.005. Issued 19 November 1985. https://patents.google.com/patent/US4554005A/en. Accessed on February 4, 2023.
Landels, S.P., A. Leder, and N. Takei. 1990. "Controlled release fertilizers." Chemical economics handbook. SRI (Stanford Research Institute) International, Menlo Park, CA. https://www.spglobal.com/commodityinsights/en/ci/products/controlled-and-slow-release-chemical-economics-handbook.html. Accessed on February 4, 2023.
Murray, T.P., C.H. Darrah III, and J.G. Clapp Jr. 2007. "Vapor pressure osmometry for prediction of turf burn from Foliar Fertilization." Communications in Soil Science and Plants Analysis 38(3–4): 337–346. https://doi.org/10.1080/00103620601172324. Accessed on January 15, 2023.
Widders, I.E. 1991. "Absorption and translocation of foliar applied trazone-n as compared to other nitrogen sources in tomato." Journal of Plant Nutrition 14 (10): 1035–1045. https://doi.org/10.1080/01904169109364263. Accessed on January 15, 2023.