The forced-use dust bag (Figure 1) has proven to be the best technique available to Florida cattle producers to manage external parasites on cattle. Forced-use dust bags are:
- Safe
- Economical
- Effective
These three qualities give dust bags a distinct advantage over other types of external parasite control in Florida.

Credit: J. F. Butler, UF/IFAS
External Parasites of Cattle That Can Be Managed with Forced-Use Dust Bags
The horn fly is one of the most serious and injurious pests of cattle (Figure 2). In Florida alone, losses due to the horn fly are estimated to total 40 million dollars per year.

Credit: J. F. Butler and P. E. Kaufman, UF/IFAS
Horn flies pierce the skin of cattle to feed on their blood. They may take up to 20 meals per day. The irritation and blood loss cause reduced weight gain of 0.3 to 0.5 lbs. per day, and horn fly infestations may lower milk production in dairy animals. Large populations of horn flies may cause open sores on the head and belly midline, which can predispose the animals to secondary infections of disease-causing pathogens. Because of their piercing-sucking mouthparts, horn flies are known vectors of Staphylococcus aureus mastitis and are suspected of mechanically transmitting anthrax and other pathogens that cause disease within herds.
Horn fly numbers of 100 or more per animal are considered to be of economic importance on dairy cattle. Beef cattle can tolerate up to 200 flies per animal before production is adversely affected. Extreme numbers of 10,000 to 20,000 flies per animal have been reported and could make blood loss alone (0.5 gal/month) an important factor in reduced production.
Horn flies can be successfully managed with forced-use dust bags. See ENY 288, Horn Fly Management, for more information on horn fly biology and control.
The cattle tail louse is the most important and damaging louse in Florida (Figure 3). The cattle tail louse is a blood-sucking louse, and extensive infestations may cause anemia in cattle. Infested cattle show poor condition, slower weight gain, low vitality, and reduced milk production. Heavy infestations of sucking lice can cause abortion and anemia in animals, and louse infestations may have been responsible for abortions in about 400 head of cattle in Putnam County (Florida).

Credit: J. F. Butler, UF/IFAS
Adult populations of more than five lice may cause economic damage to cattle. Monitoring for lice involves routine inspection of cattle. Adult lice are large, approximately 5 mm long and dark brown. They are found in the tail switch (Figure 3). Eggs are laid in the tail, at the base of the hair close to the skin. Immature stages may be seen feeding on the vulva and eyelids. Infestations of cattle tail lice, unlike those of most other cattle lice, are heaviest in the summer months.
Tail louse control can be readily achieved by timed treatments or self-treatment with proper insecticides. Although tail lice may be present year-round, it is best to treat in the months from spring to fall. Proper control procedures in the fall will prevent the winter build-up of eggs and subsequent damage to cattle when the nymphs emerge. Early spring insecticide applications will control the damaging emergence of nymphs from the over-winter build-up of eggs as well as aiding in horn fly control. Mid-winter spray treatments are not economically feasible because the population is generally in the egg stage and will not be killed by an insecticide application.
See ENY 271, Cattle Tail Lice, for more information on tail lice biology and control.
Late spring and summer use of dust bags will give excellent control of tail lice as well as horn flies. This optimum timing of appropriate pesticides can result in the control of multiple pests for the cost of one insecticide treatment.
Pesticide Use on Cattle
Cattle producers should be aware that some cattle breeds or individuals may react adversely to certain pesticides or materials contained in pesticide formulations. For example, Brahman cattle are sensitive to some organophosphate pesticides. Sensitive animals should be treated with care. If the animal's sensitivity is not known complete a test by treating a small area of skin and observe for 24 hours before treatment of the animal. The age, size, and condition of an animal may also affect sensitivity. Young animals are generally smaller than older animals and consequently need lower doses of pesticides. Always check the label to determine whether application to young animals is permitted. Unhealthy animals are also more sensitive to pesticides than those in peak condition. The additional stress of a pesticide application can kill animals that are already stressed or diseased.
In general, organophosphate and carbamate pesticides are more toxic to warm-blooded animals, and the use of many of these pesticide formulations has recently been restricted. Pyrethroid formulations are readily available and are relatively safe for use on cattle provided the proper precautions are followed. Dust formulations are generally less toxic than emulsifiable formulations and are easier to remove should toxicity problems develop. As with any pesticide, considerable restrictions exist when treating lactating dairy cattle and the label should be followed (see Table 1 for chemicals that can (and cannot) be used with lactating cattle).
If a toxicity reaction to a pesticide occurs then the pesticide formulation should be removed from the animal as soon as possible. Start by rinsing the pesticide off the skin with running water and then wash the animal with warm soapy water (room temperature). Do not scrub the skin as this increases blood circulation and the chance of breaking the skin, which is likely to enhance pesticide absorption.
See ENY-272, Pesticide Safety Around Animals, for more information about the safe use of pesticides on livestock.
Dust Bags for Self-Treament of Cattle
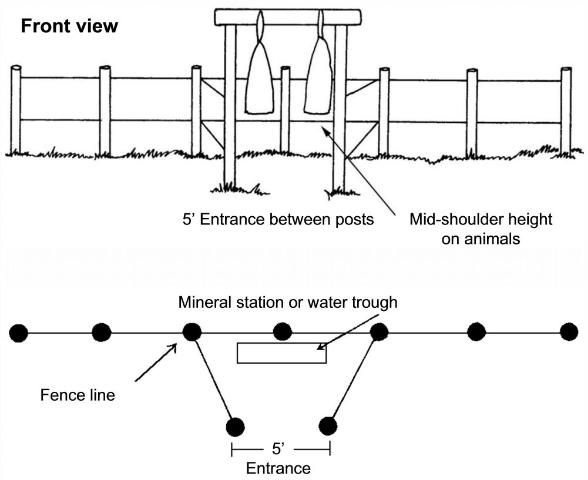
Dust bags are an effective method of horn fly and louse control. However, dust bags are only effective when hung in places where cattle are forced to use them. The best locations are areas where cattle must pass once or twice a day, or every other day, for instance between mineral boxes or water and pasture.
Dusting stations should be well-constructed and properly maintained to provide effective ectoparasite control. One dusting station with two bags is sufficient for treating approximately 50 to 60 head of cattle. Dusting stations should be constructed so that the bags hang at the mid-shoulder level (of the cow), so that the cattle must rub against the bag with their heads, sides, and backs in order to enter the station. To prevent tearing of the bags during use, cover or remove all sharp objects such as nails and barbed wire that might come into contact with the bag and tear it. Horned cattle have been known to tear dust bags, but it occurs infrequently and does not present a major problem. Dusting stations should be kept in good repair and regularly monitored. Old and worn materials should be replaced as soon as possible.
Directions for Hanging Dust Bags
Dust bags may be purchased as kits or constructed from materials readily available on the farm. Most commercial dust bags have a canvas outer bag with a burlap or nylon inner bag. Figure 4 illustrates dusting station construction and Figure 5 illustrates directions for hanging commercial dust bags. Hang the dust bags high initially so that cattle become used to passing through the station without having to push past the bags. Once the cattle are using the station, lower the bag to mid-shoulder height (of the cow). Observe cattle using the dust bags to make sure that the bag is hung at the correct height for good contact. Table 1 indicates insecticides that may be used in dust bags. Always read the label to ensure safe and effective use of pesticides.

Safety of Dust Bags
Organophosphate pesticides inhibit cholinesterase in treated animals, sometimes causing symptoms of poisoning such as excessive constriction of pupils, muscular tremors, excessive salivation, loss of reflexes, etc. Even animals not exhibiting these symptoms may not show optimum weight gain after treatment with some pesticides.
Dust formulations of a pesticide applied to Brahman and crossbred steers do not inhibit cholinesterase as strongly as the same pesticide applied as a liquid spray. Dust bags are, therefore, safer than spray formulations, especially when applied to sensitive or stressed animals. Also, there is less likelihood that control operations will decrease weight gains in treated animals.
See ENY-272, Pesticide Safety Around Animals, for more information about the safe use of pesticides on livestock.
Cost of Treatment
In the past, UF/IFAS researchers have considered the costs of various control strategies for efficient external parasite control. When available methods were ranked from least to most expensive, the three least expensive methods were applied by dust bag, which cost approximately 0.5 cents per day per animal in the 1970s. Self-application methods, such as dust bags and backrubbers, require reduced labor effort and so can be much more cost effective. Penning animals for treatment requires considerable man power and also causes stress to the animal and risks injury. Consequently, self-application strategies such as dust bags provide additional benefits.
Effectiveness of Dust Bags aganist Horn Flies
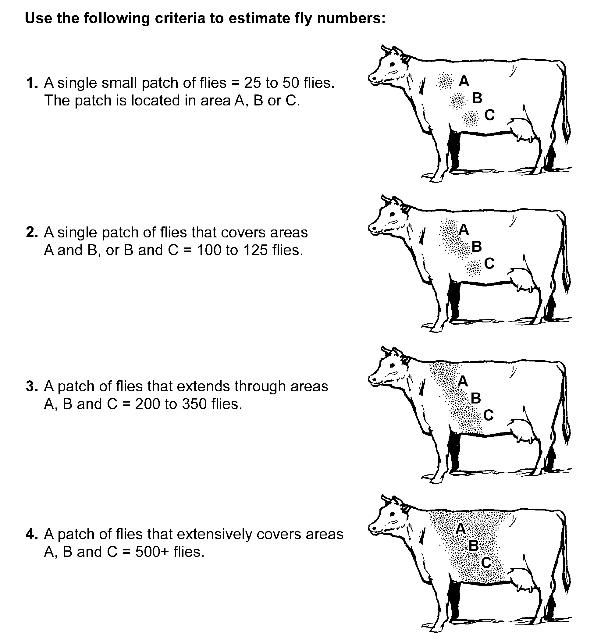
The effectiveness of dust bags has been evaluated in several studies. A study completed in Florida, in cooperation with county agents and local cattle producers, used dust bags under actual production circumstances. Producers provided two equivalent herds and placed the animals on equivalent pastures. Dust bags were hung in the fields of treated herds so that animals were forced to contact the bags to obtain water or minerals. Non-treated animals were managed according to the producers' normal practice (malathion and toxaphene spray). Weights of animals were recorded before and after treatment. Horn fly counts were recorded during the trial by county agents using the method outlined in Figure 6. In summary, during the field tests, forced-use dust bags provided an average of 90% horn fly control. Production was increased by an average of 34% over the normal management practice. This increase in production was equivalent to 1/3 lb/animal/day.
Locating an Approved Pesticide
In 2014, a group of livestock entomologists, as a part of Multistate Hatch Project S-1060, developed an online system for obtaining the names of registered pesticides appropriate for use with livestock and pets. This is a state-specific database (only certain states are represented, and Florida is one of these); if you are in another state, you must be certain that your state is represented in the dropdown list.
This database is easily searchable by the type of animal or site that you want to treat (such as a barn), as well as the targeted pest. From these two selections, you can then choose the "Method of Application" and the "Formulation Type." To use this system, please visit the following URL: http://veterinaryentomology.ucr.edu/vet_pesticides.html.
Although we continuously strive to keep this database current, it is ultimately your responsibility to ensure that the product that you choose is registered in Florida (and the application is made in Florida) and that you use the product in accordance with the label requirements and local laws and ordinances. Remember, "the label is the law" for pesticide use, and the uses indicated on the label, including the site of application and targeted pest(s) must be on the label.
If you have any challenges with this system, please contact your local UF/IFAS Extension Office (http://sfyl.ifas.ufl.edu/find-your-local-office/) or for additional assistance contact Dr. Phillip Kaufman, pkaufman@ufl.edu.
Selected References
Butler, J. F. 1985. Lice affecting livestock, pp. 101–127. In R. E. Williams, R. D. Hall, A. B. Broce and P. J. Scholl, (eds.), Livestock Entomology. Wiley, New York.
Durden, L. A. 2009. Lice (Phthiraptera), pp 56–79. In G. R. Mullen and L. A. Durden (eds.), Medical and Veterinary Entomology, vol. 2. Elsevier Science, San Diego, CA.
Matthyse, J. G. 1946. Cattle lice, their biology and control. Cornell University Agricultural Experiment Station, Ithaca. Bulletin 832.
Moon, R. D. 2009. Muscoid flies (Muscidae), pp. 45–65. In G. R. Mullen and L. A. Durden (eds.), Medical and Veteri- nary Entomology, vol. 2. Elsevier Science, San Diego, CA.
Schmidtmann, E. T. 1985. Arthropod pests of dairy cattle, pp. 223–238. In R. E. Williams, R. D. Hall, A. B. Broce and P. J. Scholl, (eds.), Livestock Entomology. Wiley, New York.
Skoda, S. R., J. B. Campbell and S. E. Kunz. 1987. Wide-area treatment of cattle for horn flies and face flies (Diptera: Muscidae) in South-central Nebraska. J. Econ. Entomol. 80: 811–816.
Steelman, C. D., R. W. McNew, R. B. Simpson, R. W. Rorie, J. M. Phillips and C. F. Rosenkrans Jr. 2003. Evaluation of alternative tactics for management of insecticide-resistant horn flies (Diptera: Muscidae). J. Econ. Entomol. 96: 892–901.
Suarez, V. H., A. L. Lifschitz, J. M. Sallovitz and C. E. Lanusse. 2003. Effects of invermectin and doramectin faecal residues on the invertebrate colonization of cattle dung. J. Appl. Entomol. 127: 481–488.
Tarry, D. W. 1985. Cattle fly control using controlled-release insecticides. Vet. Parasit. 18: 229–234.
Townsend. L. and F. W. Knapp. 2008. Insecticide dust bags for cattle insect control. University of Kentucky College of Agriculture Cooperative Extension Service. http://www.ca.uky.edu/entomology/entfacts/entfactpdf/ef515.pdf (27 August 2012).
Townsend, L. and P. Scharko. 1999. Lice infestation in beef cattle. Comp. Cont. Educ. Pract. Vet. 21(suppl.): S119-S123.
Williams, R. E. 2010. Management of cattle pests (range, confinement, dairy), pp 213–242. In R, E, Williams (ed.), Veterinary Entomology: Livestock and companion animals, CRC Press, Florida.
Wright, R. E. 1985. Arthropod pests of beef cattle on pastures and range land, pp. 191–206. In R. E. Williams, R. D. Hall, A. B. Broce and P. J. Scholl, (eds.), Livestock Entomology. Wiley, New York.