The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Frankliniella bispinosa Morgan is a common flower thrips species native to Florida and southern Georgia. Often found in association with other flower thrips, Florida flower thrips is considered to be of secondary importance relative to the more invasive western flower thrips, Frankliniella occidentalis. Nonetheless, Florida flower thrips can attain large populations quickly following flowering of susceptible crops, especially before predator populations build up. This species causes significant economic damage to a range of crops, including citrus, blueberries, strawberries, and field pepper. Field identification of different flower thrips species is difficult without expert help.

Credit: Lyle Buss, UF/IFAS

Credit: Jeff D. Cluever, UF/IFAS
Synonymy
After Hoddle et al. (2012), PaDIL (2015) and Watson (1923)
- Euthrips projectus Watson
- Euthrips masoni Watson
- Euthrips tritici bispinosa Morgan
- Frankliniella cephalica masoni
- Frankliniella tritici bispinosa Morgan
Distribution
Florida flower thrips occurs in the southeastern US and has been reported throughout Florida, southern Georgia, Bermuda, and the Bahamas, but is not known to occur in California (Hoddle et al. 2012).
Description
Flower thrips undergo partial metamorphosis, developing through five distinct stages: egg, larva I and II, pupa I (propupa) and II (pupa), and adult.
Eggs
The eggs are kidney-shaped, pale yellow, and approximately 0.4 mm (0.015 in) long. Eggs are inserted into plant tissues.
Immatures
Larvae are yellow and elongate-oval, resembling adults without wings. Pupae are non-feeding and contain wing buds, which are longer pupa II. Propupa's antennae are curved backward (posteriorly) over the head—a distinct characteristic feature compared to the pupa's antennae (pointing anteriorly).
Adults
Both sexes of Frankliniella bispinosa possess two pairs of narrow, feathery wings. The body is elongate, approximately 1 mm (0.039 in) in length, typically with the female slightly larger. The body and legs are yellow, with brown setae (hairs). Antennae are eight-segmented with stout, brown spines on the second segment.
Like the immature stages, Frankliniella bispinosa adults resemble other Frankliniella species, and are commonly found in association with Frankliniella triciti and Frankliniella occidentalis. This species is particularly similar to a Caribbean species, Frankliniella cephalica; the only recorded difference being the shape of the pedicel ring on the third antennal segment. Several diagnostic features are provided below.
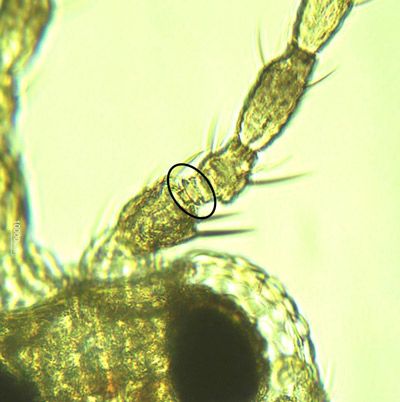
Credit: Moh Leng Kok-Yokomi, UF/IFAS
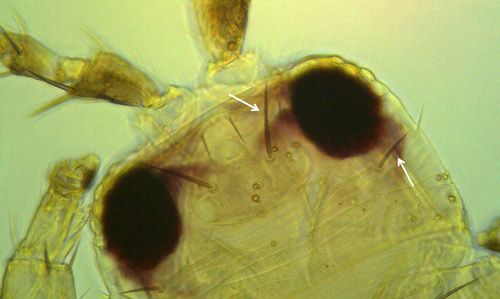
Credit: Moh Leng Kok-Yokomi, UF/IFAS

Credit: Moh Leng Kok-Yokomi, UF/IFAS

Credit: Moh Leng Kok-Yokomi, UF/IFAS

Credit: Moh Leng Kok-Yokomi, UF/IFAS
Life Cycle
Like other flower thrips, adult Florida flower thrips are highly mobile and anthophilous (seeking out and reproducing in flowers), although they also feed on developing fruits when populations are high. Mated females produce offspring of both sexes, while unmated, parthenogenetic females produce only males (Ananthakrishnan 1993). The insect feeds during both larval and adult instars, but the propupa and pupa are found in the soil and do not feed. The Florida flower thrips infests a wide range of crops and uncultivated hosts. During the summer in southeastern Florida, flower thrips can attain maturity in 11 days and have more than 10 generations per year (Childers and Nakahara 2006). The adults may live for several weeks, feeding primarily on flower tissues and pollen. Flower thrips often migrate into cropping systems from adjacent vegetation following the blooming patterns of their host plants (Frantz and Mellinger 1990). Flower thrips are naturally controlled by various predators, especially the minute pirate bug, Orius spp. Protecting these predators is an important part of integrated pest management (IPM) for flower thrips. Ramachandran et al. (2001) showed that adult Florida flower thrips moved more rapidly in field peppers compared to western flower thrips, which may allow them to better escape predation.
Hosts
Florida flower thrips infests a wide range of agricultural crops including citrus, blueberries, tomato, pepper, eggplant, cucumber, watermelon, squash, beans, strawberry, sweet corn, as well as ornamentals (Fisher and Davenport 1989, Frantz and Mellinger 1990, Childers and Nakahara 2006, Rhodes et al. 2012, Tyler-Julian et al. 2014). In addition to agricultural commodities, Florida flower thrips has been recovered from trees including Quercus, Pinus, palms including Phoenix and Sabal, and various other flowering plants such as wild cherry, southern cattail, and flowering weeds such as Bidens and Aster (Childers and Beshaer 1992, Chellemi et al. 1994, Tsai et al. 1996). Adult flower thrips may occur and feed in flowers of numerous plant species, but only a smaller sub-set of these plants serve as true reproductive hosts for flower thrips (Northfield et al. 2008).
Economic Importance
Flower thrips damage crops through feeding and oviposition, which result in deformation of growing tissues and lead to yield losses in harvested fruits and vegetables, as well as esthetic damage to flowers (Lewis 1997). Florida flower thrips is an economic pest of citrus (Childers and Nakahara 2006), blueberries (Rhodes et al. 2012), field peppers (Tyler-Julian et al. 2014), and ornamentals including chrysanthemum (Frantz and Mellinger 1990). High numbers of flower thrips migrating from post-bloomed citrus groves can inundate nearby vegetable crops. This species also infests crape myrtle in the landscape, appearing to prefer light-colored flowers (Funderburk et al. 2015). The authors confirmed a report of a severe Florida flower thrips infestation in a rose garden in St. Johns County, Florida, where light-colored blooms were preferentially infested and apparently became dwarfed. Flower thrips can be differentiated from the smaller chilli thrips, Scirtothrips dorsalis Hood, which are common pests of roses in Florida, based on their larger size as adults.
Laboratory studies have confirmed Florida flower thrips is also a capable vector of the Tomato spotted wilt virus (TSWV) tospovirus (Avila et al. 2006), although it does not appear to be a major cause of TSWV transmission in field vegetables when compared with western flower thrips (Funderburk 2009). When swarming, Florida flower thrips is known to bite people (Childers et al. 2005). Such biting does not result in any known disease transmission, but skin irritations are known to occur.

Credit: Hugh A. Smith, UF/IFAS

Credit: Wayne Myers, Jacksonville Florida Rose Society

Credit: Wayne Myers, Jacksonville Florida Rose Society
Management
Integrated Pest Management approaches, including monitoring, species identification, treatment thresholds, and reduced risk insecticides, are recommended for controlling flower thrips. Florida flower thrips may be monitored with flower tap samples and blue sticky cards (Childers and Brecht 1996). An action threshold of 100 Florida flower thrips per sticky trap per week has been proposed as a threshold in blueberries (Rhodes et al. 2012); while 10 or more adult Florida flower thrips per flower can be tolerated in tomato plants before treatment is recommended (Funderburk 2009). Plastic mulches that reflect UV are used as repellents to prevent colonization of field-grown fruiting vegetables, although the effectiveness of this approach declines as the plants grow, covering the mulch (Demirozer et al. 2012, Tyler-Julian et al. 2014).
Many insecticides are labelled for flower thrips control in vegetables and ornamental plants. However, the use of broad-spectrum insecticides may favor the buildup of western flower thrips, which is more tolerant compared with their predators and competitor native flower thrips leading to more severe pest problems (Funderburk 2009). In fruiting vegetables (peppers and tomatoes) Florida flower thrips and native species, Frankliniella triciti, are far less damaging when compared with the invasive western flower thrips or melon thrips, Thrips palmi Karny. For this reason, limiting insecticides to those compatible with natural enemies and preserving populations of less damaging native competitor thrips are recommended (Pani et al. 2008, Funderburk 2009).
Managing Thrips in Pepper and Eggplant
Selected References
Ananthakrishnan TN. 1993. Bionomics of thrips. Annual Review of Entomology 38: 71-92.
Avila Y, Stavisky J, Hague S, Funderburk J, Reitz S, Momol T. 2006. Evaluation of Frankliniella bispinosa (Thysanoptera: Thripidae) as a vector of the Tomato spotted wilt virus in pepper. Florida Entomologist 89: 204-207.
Chellemi DO, Funderburk JE, Hall DW. 1994. Seasonal abundance of flower-inhabiting Frankliniella species (Thysanoptera: Thripidae) on wild plant species. Environmental Entomology 23: 337-342.
Childers CC, Beshear RJ. 1992. Thrips (Thysanoptera) species associated with developing citrus flowers in Florida and a key to adult Terebrantian females. Journal of Entomological Science 27: 392-412.
Childers CC, Brecht JK. 1996. Colored sticky traps for monitoring Frankliniella bispinosa (Morgan) (Thysanoptera: Thripidae) during flowering cycles in citrus. Journal of Economic Entomology 89: 1240-1249.
Childers CC, Beshear RJ, Frantz G, Nelms M. 2005. A review of thrips species biting man including records in Florida and Georgia between 1986-1997. Florida Entomologist 88: 447-451.
Childers CC, Nakahara S. 2006. Thysanoptera (thrips) within citrus orchards in Florida: Species distribution, relative and seasonal abundance within trees, and species on vines and ground cover plants. Journal of Insect Science 6: 45.
Demirozer O, Tyler-Julian K, Funderburk J, Leppla N, Reitz S. 2012. Frankliniella occidentalis (Pergande) integrated pest management programs for fruiting vegetables in Florida. Pest Management Science 68: 1537-1545.
Fisher JB, Davenport TL. 1989. Structure and development of surface deformations on avocado fruits. HortScience 24: 841-844.
Frantz G, Mellinger HC. 1990. Flower thrips (Thysanoptera: Thripidae) collected from vegetables, ornamentals and associated weeds in South Florida. In Proceedings of the Florida State Horticultural Society 103: 134-137.
Funderburk C, Funderburk J, Tyler-Julian K, Srivastava M, Knox G, Andersen P, Adkins S. 2015. Population dynamics of Frankliniella bispinosa (Thysanoptera: Thripidae) and the predator Orius insidiosus (Hemiptera: Anthocoridae) as influenced by flower color of Lagerstroemia (Lythraceae). Environmental Entomology, in press.
Funderburk J. 2009. Management of the western flower thrips (Thysanoptera: Thripidae) in fruiting vegetables. Florida Entomologist 92: 1-6.
Hoddle MS, Mound LA, Paris DL. (2012). Thrips of California. CBIT Publishing, Queensland. (14 September 2015).
Lewis T. 1997. Thrips as Crop Pests. Cab International, Wallingford, UK, 740 pp.
Northfield TD, Paini DR, Funderburk JE, Reitz SR. 2008. Annual cycles of Frankliniella spp. (Thysanoptera: Thripidae) thrips abundance in north Florida uncultivated reproductive hosts: Predicting possible sources of pest outbreaks. Annals of the Entomological Society of America 101: 769-778.
PaDIL http://www.padil.gov.au. (15 September 2015).
Paini DR, Funderburk JE, Reitz SR. 2008. Competitive exclusion of a worldwide invasive pest by a native. Quantifying competition between two phytophagous insects on two host plant species. Journal of Animal Ecology 77: 184-190.
Ramachandran S, Funderburk J, Stavisky J, Olson S. 2001. Population abundance and movement of Frankliniella species and Orius insidiosus in field pepper. Agricultural and Forest Entomology 3: 129-137.
Rhodes EM, Liburd OE, England GK. 2012. Effects of southern highbush blueberry cultivar and treatment threshold on flower thrips populations. Journal of Economic Entomology 105: 480-489.
Tsai JH, Yue BS, Funderburk JE, Webb SE. 1995. Effect of plant pollen on growth and reproduction of Frankliniella bispinosa. Tospoviruses and Thrips of Floral and Vegetable Crops 431: 535-541.
Tyler-Julian K, Funderburk J, Frantz G, Mellinger C. 2014. Evaluation of a push-pull strategy for the management of Frankliniella bispinosa (Thysanoptera: Thripidae) in bell peppers. Environmental Entomology 43: 1364-1378.
Watson JR. 1923. The proper name and distribution of the Florida flower thrips. The Florida Entomologist 7: 9-11.