The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids, and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The winter ant, Prenolepis imparis (Say), is a widespread North American ant that is common across the United States. Sometimes called the false honey ant, this dominant woodland species (Fellers 1989) is most active during cool weather, when most other ant species are less likely to forage. This species is one of the few native ants capable of tolerating competition with invasive species, and it persists in areas invaded by the Argentine ant, Linepithema humile. This species accomplishes this by foraging when many invasive species are inactive, and they are also aggressive toward other ants and produce abdominal secretions that are lethal to Linepithema humile (Sorrells et al. 2011). Prenolepis imparis is ecologically important as a seed disperser, and has been observed dispersing seeds of herbaceous plants in the southern Appalachian Mountains (Gaddy 1986).
Synonymy
Formica imparis Say
Formica wichita Buckley
Prenolepis imparis arizonica Wheeler
Prenolepis imparis californica Wheeler
Prenolepis imparis colimana Wheeler
Prenolepis imparis coloradensis Wheeler
Prenolepis imparis minuta Emery
Prenolepis imparis pumila Wheeler
Prenolepis imparis testacea Emery
Prenolepis imparis veracruzensis Wheeler
Prenolepis nitens americana Forel
Distribution
Prenolepis imparis is the only Prenolepis species in the New World; its closest relatives occur on other continents. This species is found throughout North America, from southern Ontario to northern Florida and south into Mexico (Wheeler 1930). Its geographic range spans from the east to the west coast of the United States (Figure 1). In Florida, it is found as far south as Citrus and northern Orange counties and throughout the panhandle (Figure 2) (Deyrup 2017). Its sister species, Prenolepis nitens, is found in southeastern Europe and 11 other Prenolepis species occur in subtropical and tropical Asia (Williams & LaPolla 2016). There is also one fossil species, Prenolepis henschei, known from mid-Eocene (about 44 million years old) Baltic amber (LaPolla and Dlussky 2010).
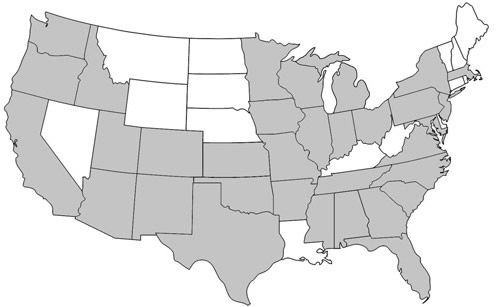
Credit: AntWiki
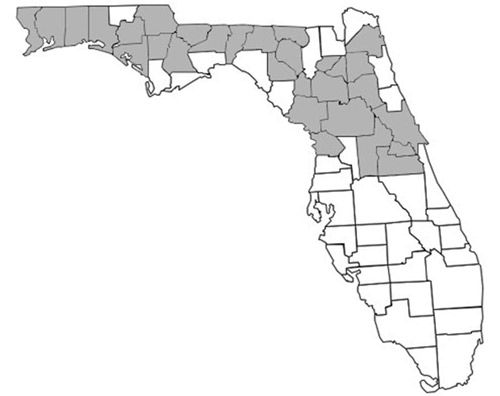
Description
Worker
Adult workers (Figure 3) are 3.0–4.5 mm long and variable in color, ranging from light to dark brown with the head and gaster (abdominal segments posterior to the petiole) sometimes darker than the lighter brown mesosoma (midsection of the body) and legs. Overall, the cuticle is smooth and shiny with no surface sculpturing. Erect hair-like macrosetae are scattered across the posterior margin of the head, mesosoma and gaster. The scape (the long, first antennal segment) and the legs are covered in decumbent setae.

Credit: Williams and LaPolla (2016)
Head
The head is approximately as broad as it is long, and relatively square in overall shape, with rounded corners. The posterior margin is straight and the posterolateral corners are rounded. The compound eyes are situated posterior to the midline of the head and are moderately convex, but do not extend beyond the sides of the head when viewed from the front. The antennae are 12-segmented and long, extending well beyond the posterior margin of the head.
Body
The most definitive diagnostic characteristic of the worker of this species is its strongly constricted mesosoma, which is defined by a strong mesonotal depression just posterior to the pronotum. When viewed from the side, the body has a shape similar to an hourglass or a peanut, with the dorsal and ventral surfaces of the mesosoma appearing to be "squeezed" together. This characteristic is a key identifying feature of the entire genus Prenolepis. Because Prenolepis imparis is the only member of the genus found in North America, identification of this species is straightforward. In profile, the petiole is forward-inclined and subtriangular in shape with an angled and pointed node. The gaster is large and rounded and often swollen from reserves stored while foraging.
Queen
Prenolepis imparis queens are 7.0–8.5 mm long and much larger than males, which are 3.0–4.0 mm long. The queens are always light to medium reddish-brown and the males are always black. The original description of this species by Say (1836) was based on observations of a reproductive pair in copula. He chose the name imparis because of the stark color contrast between the queen and male (Figure 4).

Credit: Alexander Wild, www.alexanderwild.com
Biology
Prenolepis imparis is a generalist omnivore, though it has been found to prefer a high protein and lipid diet (Lynch et al. 1980). Foragers are known for tending to aphids or scale insects (Figure 5) from which they consume excreted honeydew, aggregating on rotting fruit, and exploiting protein-rich sources such as dead annelids (Wheeler 1930, Talbot 1943, Sorrells et al. 2011). Once a rich food source is discovered, foragers quickly mobilize and become highly aggressive in defending it from competitors (Lynch et al. 1980). While most ants tend to forage at warmer temperatures, Prenolepis imparis is most active at air temperatures between 45° and 60°F (Talbot 1943) and has been observed foraging at temperatures near freezing (Wheeler 1930). During the warmest months of the year, nest entrances are sealed and the colony enters an estivation period with no presence aboveground. Seasonal foraging activity and estivation of Prenolepis imparis varies with latitude, with colonies in the northern part of the range being active throughout the year with the exception of the summer months (Talbot 1943). Activity is different in southern populations, with Florida foragers active only between November and early April (Tschinkel 1987).

Credit: Alexander Wild, www.alexanderwild.com
Life Cycle
Like all other ants, Prenolepis imparis is holometabolous (undergoes complete metamorphosis) and has the following life stages (in order): egg, larva, pupa, and adult. The eggs are small, white, and cylindrical (Figure 7). Pupae are sometimes erroneously called "ant eggs", but can be distinguished by being encased in silk cocoons and by their size, which is much closer in size to the adults, whereas eggs are much smaller. Larvae (Figure 6A-C) are maggot-like, plump, slightly curved, and covered in two types of hairs: (1) simple (Figure 6D); and (2) two- to three-branched (Figure 6E-F) (Wheeler and Wheeler 1953).
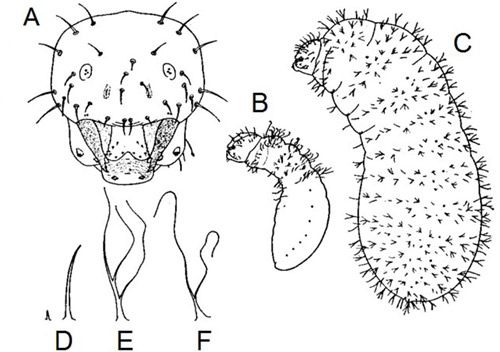
Credit: Modified from Wheeler and Wheeler (1953)

Credit: Alexander Wild, www.alexanderwild.com
Colonies are polygynous and typically have a few thousand workers, but may include over 10,000 individuals in colonies that are 7 to 9 years old (Tschinkel 1987). A single brood consisting of both workers and reproductives is produced annually in late August to September. The reproductives overwinter in the colony and are released for their nuptial flight in the early spring; usually between late February and early April. Prenolepis imparis is the first of all North American ant species to form mating swarms in the spring (Wheeler 1930), which tend to aggregate on low-lying vegetation and on the trunks of trees.
Young adult workers eclose in autumn and spend the next 10 months inside the colony, while older workers forage outside the nest and return with liquid food. The foragers pass their collective food reserves via mouth-to-mouth regurgitation to the young workers in a process called stomodeal trophallaxis. Young workers have softer, more flexible cuticles and their gasters can swell and distend to accommodate large amounts of liquid food (Figure 6). Wheeler (1930) and Talbot (1943) thought that these swollen workers were repletes, which store liquid carbohydrates in their crops, as honeypot ants do (Myrmecocystus spp.). However, Tschinkel (1987) described them as "corpulents" when he discovered that the distention of their gasters was actually caused by the storage of lipids in hypertrophied fat bodies. Corpulents store more than twice their body weight in fats and nutrients. Unlike true honeypot ant repletes, the corpulent state seen in Prenolepis imparis is reversible; once a corpulent worker has exhausted its food stores it becomes a forager during the next active season. This indicates that workers may live for up to two years, serving as a corpulent for the first and a forager during the second.
Nest Architecture
The nest architecture of Prenolepis imparis colonies in northern Florida has been studied extensively by Tschinkel (1987, 2011). Nest entrances are commonly found in shaded areas near the bases of trees and are characterized by having a series of short dead-end tunnels nearby, presumably for foragers to take temporary shelter from predators or the elements during cold weather. Nests in Florida have been recorded extending as far down as 3.6 meters. From the entrance, the main shaft of the nest extends vertically downward, with no chambers found shallower than 60.0 cm, after which point chambers are very common and are all directly connected to the main shaft (Figure 8). The shallowest and deepest chambers are the smaller ones, with larger ones in between. Density of individuals within chambers directly increases with depth. Young and corpulent workers are most common in the deepest chambers. One explanation for the extreme nest depth is that it facilitates cold weather activity, since temperature deep in the soil is much less variable than that of the surface.

Credit: Tschinkel (2011)
Nest depth is variable across geographic range and substrate type (Tschinkel 1987). The tunnels of Prenolepis imparis colonies in northern Florida are especially deep, stretching down nearly three times deeper than nests surveyed in Missouri and Ohio (Talbot 1943) and four times deeper than those in Tennessee (Dennis 1941). This could be due to the sands of Florida being easier to excavate than the clays in the other areas, but temperature differences between Florida and the more temperate north may also play a role. Despite differences in depth between the surveyed nests the overall structure is about the same, with chambers occurring much shallower in the nests outside of Florida.
An unusual case of an unseasonal mating flight occurring in the Sierra Nevadas, California in October, 2001 was observed by Alex Wild (pers. comm. September 9, 2015). Two queens were collected, one of which is imaged on AntWeb.org (Figure 9). The queens appear to be of a Prenolepis species but are much smaller and with less pilosity than those of Prenolepis imparis. These may be smaller Prenolepis imparis queens (called "microgynes") or those of an undescribed species. More specimens and further differences will be necessary to determine if this is truly a distinct species (Williams and LaPolla 2016).

Credit: AntWeb
Economic Importance
Prenolepis imparis is not a pest species, though foragers and winged reproductives have been known to occasionally enter homes and other buildings. Pesticides are not needed and should not be used to manage this species. Instead it is recommended to seal any possible entryways, especially around doors and windows, to prevent them from entering.
Selected References
Anonymous. (2017). Prenolepis imparis. AntWiki. (5 January 2017)
California Academy of Sciences. (2017). Specimen: CASENT0005430 Prenolepis ca01. AntWeb. (5 January 2017)
Dennis CA. 1941. Some notes on the nest of the ant, Prenolepis imparis Say. Annals of the Entomological Society of America 34: 83–86.
Deyrup M. 2017. Ants of Florida: Identification and Natural History. Boca Raton: Crc Press.
Fellers JH. 1989. Daily and seasonal activity in woodland ants. Oecologia 78: 69–76.
Gaddy LL. 1986. Twelve new ant-dispersed species from the southern Appalachians. Bulletin of the Torrey Botanical Club 113: 247–251.
Lapolla JS, Dlussky GM. 2010. Review of fossil Prenolepis genus-group species (Hymenoptera: Formicidae). Proceedings of the Entomological Society of Washington 112: 258–273.
Lynch JF, Balinsky EC, Vail SG. 1980. Foraging patterns in three sympatric forest ant species, Prenolepis imparis, Paratrechina melanderi and Aphaenogaster rudis (Hymenoptera: Formicidae). Ecological Entomology 5: 353–371.
Say T. 1836. Descriptions of new species of North American Hymenoptera, and observations on some already described. Boston Journal of Natural History 1: 209–305.
Sorrells TR, Kuritzky LY, Kauhanen PG, Fitzgerald K, Sturgis SJ, Chen J, Dijamco CA, Basurto KN, Gordon DM. 2011. Chemical defense by the native winter ant (Prenolepis imparis) against the invasive Argentine ant (Linepithema humile). PLoS ONE 6: e18717.
Talbot M. 1943. Population studies of the ant Prenolepis imparis Say. Ecology 24: 31–34.
Tschinkel WR. 1987. Seasonal life history and nest architecture of a winter-active ant, Prenolepis imparis. Insectes Sociaux 34: 143–164.
Tschinkel WR. 2011. Subterranean ant nests: trace fossils past and future? Palaeogeography, Palaeoclimatology, Palaeoecology 192: 321–333.
Wheeler WM. 1930. The ant Say. Annals of the Entomological Society of America 23: 1–126.
Wheeler GC, Wheeler JN. 1953. The ant larvae of the subfamily Formicinae. Annals of the Entomological Society of America 46: 126–171.
Williams JL, LaPolla JS. 2016. Taxonomic revision and phylogeny of the ant genus Prenolepis (Hymenoptera: Formicidae). Zootaxa 4200: 201–258.