Foliar or bud nematodes (Aphelenchoides spp.) were until recently largely unknown to strawberry growers in Florida, and very little is known about their biology and behavior in the state. When present, foliar nematodes are capable of causing significant crop loss in strawberries.
History
Plant-parasitic nematodes are small roundworms that cause tremendous economic damage in agriculture and horticulture. Their microscopic size and transparent nature make them difficult to detect and to identify. Most nematodes occur in soil or in subterranean plant parts where they feed on living plant tissues, often resulting in below- and above-ground symptoms such as root galls, lesions, stunting, wilting, or chlorosis. Most plant-parasitic nematodes parasitize roots, but some nematodes, including certain species of Aphelenchoides nematodes, can feed on aboveground plant parts.
The earliest evidence of foliar or bud nematodes in Florida dates back to 1901, when a disease called "crimp" was described on strawberries. Crimp was probably identical with what was called "dwarf" disease in Louisiana and the "red plant" disease in Great Britain. Other names that were used for the disease were French bud, white bud, red bud, briar bud, possum's ear, male plant, and wild plant. In the early 1900s crimp was the most widespread of any of the major diseases of strawberry in Florida. Early research on this disease in Florida was done by Albert Brooks, a plant pathologist at the Strawberry Investigations Laboratory at Plant City. He was the first one to describe the disease in Florida in 1931 and called it "strawberry crimp." Brooks found high numbers of nematodes in diseased buds, often up to more than 1000 nematodes per bud, but none in healthy buds. He was also able to transfer the disease into healthy plants by inoculating these with nematodes. Transfer to healthy plants of other pests such as strawberry aphids that were present on crimped plants did not produce the disease. Brooks identified the foliar nematodes as Aphelenchus fragariae (now Aphelenchoides fragariae). Foliar nematodes had been known since the 1890s from begonia and were initially named Aphelenchus olesistus (Smith 1890, and Ritzema Bos 1892).
The prevalence of crimp in the early 1900s was probably related to the strawberry production system that was in place in Florida. At that time, plants imported from mainly Maryland and Arkansas were set out in February–March and allowed to make runners until June. At that time, the runner plants were transplanted to other beds, where they in turn produced runner plants for setting from September through October for fruit production, which typically started late November through May. This cycle was repeated annually. With strawberry plants in the field throughout the year, the disease could progress and be observed permanently, especially when the temperature was high and the plants were putting on new growth, most prominently during the rainy season, July through September. Crimped plants were considered worthless as fruit producers because the fruit they put on was late and of inferior quality. The loss of plants due to crimps ranged from 0–75% in individual fields and about 2% for the entire state. Crimp was also reported in Arkansas, Tennessee, and North Carolina at that time, but not in Maryland.
From the 1930’s until the 1960’s, crimp was frequently observed in commercial FL strawberry nurseries. Years when crimp did not show up were linked to low spring temperatures, setting of plants in new ground, and careful selection of nursery plants. There is no certainty about which species of Aphelenchoides caused crimp in Florida during those days. During the early years they were identified as A. fragariae, but in later reports starting around the 1950’s there is no more mention of A. fragariae, instead the nematodes were now identified as A. besseyi. A. besseyi was first described in 1942 by J.R. Christei, so possibly the earlier reports were misidentifications. Very few reports exist on bud nematodes in FL strawberries after the 1970’s. In 2014 the nematodes were reported from one farm (Noling, personal communication), and in 2016-17 they were found on six farms. The 2016-17 foliar nematodes were identified morphologically as A. besseyi (R. Inserra, FDACS, DPI, Gainesville, (R. Inserra, DPI, Gainesville, FL) (Figure 1). This identification was confirmed by molecular analyses of topotype populations of this species (Oliveira et al, 2019). Several other species of Aphelenchoides have since been identified from Florida strawberry fields, including a newly described species A. pseudogoodeyi which appears to be fungal-feeding and not cause damage to strawberry (Oliveira et al, 2019; Subbotin et al, 2020).
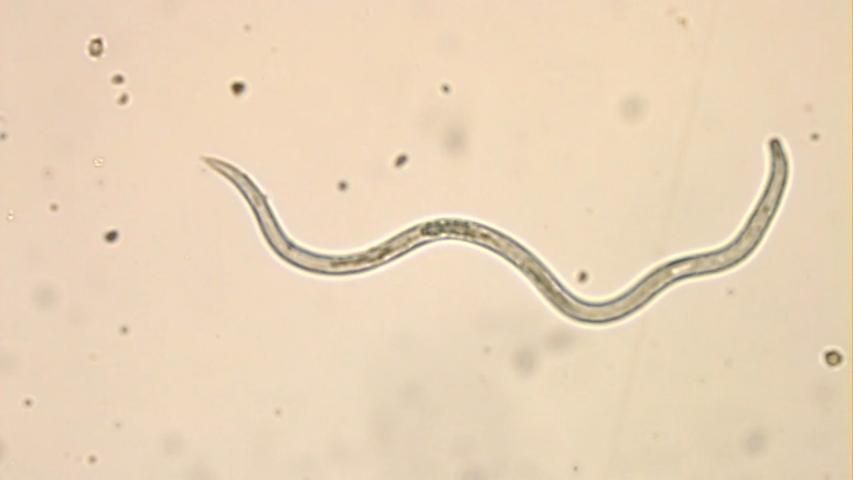
Main Species and Hosts
Nematodes in the genus Aphelenchoides occur commonly throughout the world. Most of them live in soil and feed exclusively on soil fungi. Of the more than 220 known species in the Aphelenchoides genus, only a few are facultative plant parasites. The most important plant-parasitic species are Aphelenchoides besseyi, A. fragariae, and A. ritzemabosi (Table 1). A besseyi is limited to tropical and subtropical regions, whereas the other two species are more commonly found in temperate regions (GBIF 2016).
Important hosts of A. fragariae and A. ritzemabosi are strawberries, ferns, tropical foliage plants, and vegetatively propagated ornamentals. They have been reported on more than 700 plant species in 85 families that include both herbaceous and woody plants. Some examples of hosts are onion, garlic, sweet corn, sweet potato, soybean, chinese cabbage, sugar cane, horseradish, lettuce, millet, many grasses, orchids, wishbone, chrysanthemum, marigold, mexican sunflower, african violets, rubber plant, Hibiscus, hydrangea, and many grasses (Panicum, Pennisetum, and Setaria).
Aphelenchoides besseyi is the most tropical of the foliar nematodes and is best known as the causal agent of rice white-tip disease (Figure 2). White-tip disease of rice (Oryza sativa L.) was first described by Kakuta in Japan in 1915. Because the nematode can be transmitted via seeds, the disease can now be found in all rice-growing regions in the world. The nematode causes a characteristic whitening of the leaf tips, which later become necrotic, and results in reduced grain size. Symptoms may be confused with calcium and magnesium deficiency. A. besseyi also infests strawberries, where it is the causal agent of "summer dwarf" or "crimp" disease, a disease recorded from the United States, Australia, and Europe. Symptoms include leaf crinkling and distortion, and dwarfing of the plant, with an associated reduction in flowering. Symptoms may be confused with those caused by the two other foliar-feeding Aphelenchoides species.
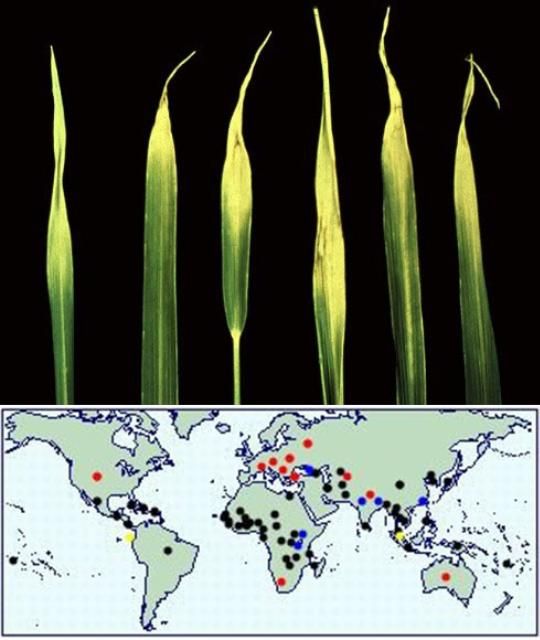
Credit: EPPO (2016), and CABI (2017)
Life Cycle and Feeding Behavior
Aphelenchoides like all nematodes, are non-segmented roundworms with six life stages: an egg stage, four larval or juvenile stages, and an adult stage. Like all plant-feeding nematodes they have a needle-like, hollow spear mouth part (or stylet) that is pushed into the plant cell. The worm forces enzymes through the stylet into the cell where cell components are digested and then drawn back into the nematode's digestive system through the stylet. Adult foliar nematodes are about 0.04 inches (1 millimeter) long, and reproduce by laying eggs, which hatch to release a larva. The nematodes mature through a series of molts as the larvae enlarge and become adult worms. Sexual reproduction is required for fertile offspring, but fertilized females can store sperm in a specialized organ to allow continued reproduction for months without additional fertilization. The females deposit 25 to 30 eggs in a compact group, and within 3 to 4 days, after the first molt inside the egg, the second-stage juvenile hatches. Reproduction is prolific, and they complete their life cycle (from egg to adult) in 8–14 days, allowing many generations to develop during one growing season (several thousand nematodes can occur in a single infected leaf).
Optimum temperatures for foliar nematode development are between 21 and 24°C (70–75°F) for the temperate species (A. fragariae, A. ritzemabosi), and 30°C (86°F) for the tropical A. besseyi. No development of A. besseyi was reported at temperatures below 13 °C (55 °F) (Ferris 2017).
Leaf nematode problems are usually restricted to high-humidity environments because the nematodes require a thin water-film on the stems and leaves in order to move. Foliar nematodes mostly feed outside the plant tissue by inserting their hypodermic syringe-like mouth stylet into cells to withdraw the contents, causing the leaves, especially the new growth, to appear curled, twisted, and stunted. However, when low populations of nematodes are present there may not be any visible symptoms. In some hosts other than strawberry, foliar nematodes can make their way inside leaf and stem tissue by penetrating the tissue through the stomata, the pore openings in the leaf surface through which gas and water vapor exchange occurs (Figure 3). They then move between the spongy mesophyll cells, feeding and causing the cells to die and discolor. This has been observed in Ficus elastica and Polianthes tuberosa, in which it causes leaf drop and leaf lesions, respectively. On Capsicum annuum, the infestation appears to result in rotting of the pods and premature pod drop.
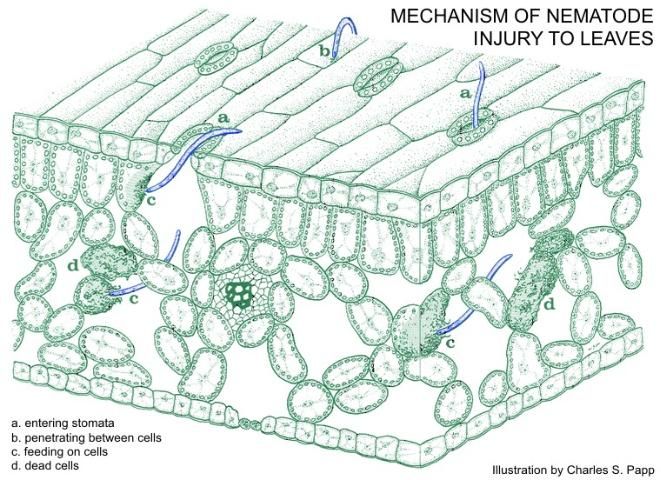
Credit: Meyer (2017)
The genus Aphelenchoides are mostly fungus feeders. Only a few parasitize plants, and they are all facultative plant parasites. The most important of these are the three species mentioned earlier, Aphelenchoides besseyi, A. fragariae, and A. ritzemabosi. A facultative parasite is one which, when no host is available, can nevertheless survive. These nematodes do not need a plant host. They can also feed and survive on fungi, either in the soil or on decaying plant material.
Damage Symptoms
Foliar nematode damage can be confused with damage caused by bacteria, fungi, viruses, nutrient deficiencies, or chemical injuries. Nematodes may also interact with certain fungi or bacteria to cause severe foliar blight. In strawberries, mites or thrips can cause damage symptoms that look similar to nematode damage symptoms.
Foliar nematodes occur mainly within the leaves and crowns of their hosts, where infested buds, young leaves, or shoots typically become deformed and will not develop properly. Plants will often remain undersized, become bushy or distorted, and produce little or no marketable foliage or flowers. In strawberry, young plants are often dwarfed and present a spider-like appearance. Leaves appear crimped, twisted, or crinkled and are a darker green than normal (Figure 4, left). A reddish color may appear on the leaf edges and veins, and leaves tend to become more brittle, with tips of young leaves and stipules of older plants drying up and turning brown. Petioles are often short, thick, less pubescent than those of unaffected plants, and have a reddish cast. Damaged plants put on fruit late, and it is often of inferior quality, sometimes showing "phyllody" or "broccoli fruit" (Figure 4, right).

Credit: J. Desaeger
Most nematodes in strawberry plants are found in the protected spaces at the bases of the young leaves and in the folds of the leaves in the bud. Brooks (1931) could artificially produce crimps by placing some of these nematodes in the buds of healthy plants. The time required for the symptoms to appear varied from 11 to 120 days, depending upon temperature, moisture, and whether or not the plant was putting on new foliage.
In the 2016–17 strawberry season, we observed a striking change in plant habit of nematode-infected strawberries throughout the growing season. Early in the season, A. besseyi-infected plants showed the typical crinkling and folding of leaves, and plants were compact and stunted. However, by the end of the season those same plants had become larger and greener as compared to non-infected plants (Figure 5).

Credit: J. Desaeger
Although infected plants were taller by the end of the season, they produced little or no flowers and very few and mostly abnormally shaped fruits (Figure 4, right). Clearly, A. besseyi triggered a response in strawberry plants that dramatically changed its growth habit, increasing vegetative growth (its main food source) at the expense of flower and fruit production. The ability of A. besseyi to stimulate vegetative growth over growth of flowers and fruits has also been observed in a common weed, smutgrass (Sporobolus poirettii), in which the nematode was found to increase inflorescences (EPPO, 2016).
In other plants, foliar nematode symptoms often include localized chlorotic or purplish angular lesions that are delineated by leaf veins (it is difficult for the nematodes to move across the veins). This often creates a patch-like appearance on dicots and a stripe-like pattern with monocots (because monocots have parallel veins, discoloring on them looks like streaks) (Figure 6.) Discoloring typically starts near the leaf base and spreads outward. The symptoms of angular spots are often mistaken for bacterial leaf spots or fungal pathogens such as downy mildew which are also commonly confined between the leaf veins. In Costa Rica, A. besseyi has been reported to cause a disease of bean called angular leafspot (Figure 6).

Credit: W. Barrantes
Dissemination and Survival
Foliar nematodes can be disseminated in many ways. Infestation is most easily accomplished by rain or irrigation water washing the nematodes into the plant buds. Water is a major factor in helping the spread of foliar nematodes in a field because a thin film of water must be present for foliar nematodes to move across leaves and stems. Actively, nematodes will only travel slowly from plant to plant, but in fields that are irrigated overhead or inundated, their spread can become more rapid (Figure 7). Water from splashing rain and irrigation readily moves the nematodes from leaf to leaf and plant to plant.

Credit: J. Desaeger
Long-distance spread of foliar nematodes occurs when diseased runners from nursery beds are removed and reset in other locations. Recently infected plants often do not display symptoms and, thus, are transplanted as apparently healthy plants. Brooks (1931) suggested that during the propagation of plants in spring and summer, the runners formed by crimped plants usually become infected as they push through the infected areas at the base of the leaves. This resulted in circular areas of diseased plants appearing in the nursery beds.
Foliar nematodes can also infest new plants by swimming up stems and moving along moist plant tissue surfaces. In some hosts they may also enter the leaves through stomata, but this has not been observed on strawberries. Foliar nematodes can mature from egg to adult in about 2 weeks, allowing many generations to develop during one growing season. Another potential means by which foliar nematodes are spread is through the harvesting process. Especially when plants are still wet from morning dew, the nematodes can easily be spread by the harvesting crew as they drag their hands through the planting rows while picking the berries (Figure 8).

Credit: J. Noling
Foliar nematodes can also be found in soil or decomposing organic material where they feed on fungi. Their survival in soil is not thought to be long, probably a few months only. Foliar nematodes more typically overwinter in dormant buds, plant terminals, and on the soil surface in dead leaves that drop from infested plants (possibly feeding on fungi that developed on dead leaves). In slowly drying leaf tissue, adults of A. ritzemabosi can enter a desiccated resting stage that allows them to survive for several years until moist conditions allow them to resume activity. In rice, A. besseyi are able to survive in a quiescent state on infested seed for long periods of time, from 8 months to 3 years, depending on the rate of drying (Cralley 1949, and Yoshi and Yamamoto 1950).
Brooks in 1929 suggested that in Florida strawberries the disease was carried over from year to year in the soil, but there is no data to support this. At this point it is not known if A. besseyi can survive between strawberry seasons (during summer) in Florida, and if field practices during summer (presence or absence of dry strawberry material and weeds and/or double-cropping with vegetables or planting of cover crops) affects nematode survival.
The 2016 increase in occurrence of foliar nematode damage in Florida can be traced back to infected strawberry nursery transplants. Strawberry plants are shipped every season to Florida farms from nurseries in mostly Canada, California, and North Carolina. Canada is not thought to be the source because A. besseyi is a tropical nematode that has been found in North Carolina and the southeastern United States but not further north. The outbreak in 2016 was linked to one specific nursery location in North Carolina. With the loss of methyl bromide, less-effective nursery transplant pest management practices are being used, and pests and diseases in nurseries have become more difficult to control. Recently, more reports on foliar nematode problems have come to our attention, including some from Brazil where A. besseyi caused disease in soybean and forage grasses. More research on A. besseyi seems to be in order, because it is a potentially devastating nematode for a wide range of crops.
Detection and Management
Foliar nematodes can be easily diagnosed by cutting symptomatic plant tissue into small pieces, placing it in a clear glass container or on a microscope slide, and adding water to immerse the plant tissue. Allow the pieces to soak for a few minutes up to a few hours, and, using at least 10X magnification, look for small worms moving in rapid, snake-like motions. Nematodes will appear as tiny strands moving in the water. It is important to have the lighting correct and a dark background color to see the worms. The nematodes will sink to the bottom of the container over time.
There are no good management practices for heavy infestations. The key is to implement management strategies preventatively or upon early detection of nematodes. The following is an overview of the different management options for foliar nematodes.
Nematode-free plant material: production of disease-free nursery plants is the first and most important tactic to manage foliar nematodes. Outbreaks are typically traced back to infested but often asymptomatic vegetative propagation material, such as rooted cuttings or seedlings. Plants should be grown on nematode-free land, and, when using plug plants, poorly composted organic material should never be used in planting mixes unless it is treated appropriately to eliminate nematodes and other soilborne pathogens. In production nurseries, there should be zero tolerance to foliar nematodes, and any nematode occurrence must be thoroughly cleaned and disinfested. This is especially important during the propagation of plants in spring and summer because the runners formed by crimped plants usually become infected as they push through the infected areas at the base of the leaves.
Hygiene and soil-free propagation techniques—growing plants in soilless media or pasteurizing media—can greatly reduce the occurrence of foliar nematodes. Soil-free techniques and good hygiene will not prevent a problem if the nursery stock is infected, however, so growers must take care to propagate only nematode-free stock. Foliar nematodes are typically introduced into growing areas in cuttings, seedlings, and other vegetative propagation material that may be asymptomatic. When taking cuttings, take only from the tops of long, vigorous growth to reduce the likelihood that cuttings are infested.
Discard infected plants: employ good sanitation practices by removing plant debris, promptly disposing of all infested plants, and eliminating weeds that can host foliar nematodes from around growing areas. Diseased plants and old foliage should be destroyed, because even the dried-out nematodes on dead plants are potentially destructive. In slowly drying leaf tissues, foliar nematodes may be able to survive by undergoing major physiological changes and entering a state called anhydrobiosis. Nematodes in anhydrobiosis have no detectable metabolic activity, but they are still alive. Even after years of immobility, if dried-out nematodes are allowed to rehydrate, they can regain activity and get back to reproducing, eating, and destroying crops. Dried nematodes can also easily be spread by people working in a crop.
Eliminate or limit overhead watering: if possible modify the irrigation practices by limiting overhead irrigation to reduce splashing water from plant to plant and to decrease the time leaf surfaces remain wet after watering. Also, avoid crowding plants to reduce the risk that foliar nematodes will spread throughout the crop by traveling in a water film on plant surfaces.
Hot water treatment: Brooks reported in 1930 that nematodes suspended in water were killed by a temperature of 48°C (120°F) for 20 minutes or 47°C for 30 minutes and crimped plants in pots immersed in water 48°C for 20 minutes resumed normal growth afterwards. Other more recent studies on ornamentals reported that if the plants will tolerate heat without damage, cuttings can be disinfected by dipping them in hot water at 122°F (50°C) for 5 minutes or at 111°F (44°C) for 30 minutes. Timing and temperature are very important when performing heat treatments. To avoid damage to plant material, it is critical that both temperature and exposure time are accurately controlled.
Nematicides: many of the nematicides and insecticides that successfully controlled foliar nematodes in the past are no longer registered for use in the United States. This, combined with the changing soil fumigation practices in strawberry nurseries from broad-spectrum fumigants like methyl bromide to other, less effective substitutes, is likely linked to the re-emergence of foliar nematodes in Florida. The following is a list of products (insecticides, fungicides, and biologicals) that have nematicidal activity and can be used in Florida strawberries. However, at the moment there is no efficacy data on foliar nematodes available on any of these products. Products include AgriMek (a.i abamectin), Luna (a.i. fluopyram), Majestene/Venerate (a.i. Burkholderia), NemaKill (a.i. essential oils) and Nimitz (a.i. fluensulfone).
Acknowledgements
This publication is dedicated to Dr. Albert N. Brooks, who was a pioneer in the study of nematodes on strawberries in Florida. His first report in 1927 pertained to root-knot on strawberry. In 1929 he began to study "strawberry crimp disease," and in 1930 he showed that foliar or bud nematodes caused the disease. In 1931, Dr. Brooks published an article in the Florida Agricultural Experiment State Bulletins titled "Crimp – a Nematode Disease of Strawberry." Some scattered references to crimp were made later, and in 1947 Dr. Brooks reported the presence of root-lesion nematodes on strawberry. In 1948–49 he became interested in the possibility that sting nematodes caused a disease of strawberry and was instrumental in getting Dr. J. R. Christie, USDA nematologist, to investigate the strawberry problems.
References
Brooks, A.N. 1929. "Strawberries in Florida." Culture, Diseases and Insects. University of Florida Agricultural Experiment Station. Annual Report 1929, p. 500–502.
Brooks, A.N. 1931. "Crimp – A nematode disease of strawberry." University of Florida Agricultural Experiment Station. Annual Report 1931. Bulletin 235, p. 1–27.
Brooks, A.N. 1940. "Crimp – Investigations relative to certain diseases of strawberries of importance in Florida." University of Florida Agricultural Experiment Station. Annual Report 1940. Bulletin 235, p. 105.
CABI. 2017. Invasive Species Compendium. Aphelenchoides besseyi. http://www.cabi.org/isc/datasheet/6378
Christie, J. R. 1942. "A description of Aphelenchoides besseyi n.sp., the summer dwarf nematode of strawberries, with comments on the identity of Aphelenchoides subtenuis (Cobb, 1929) and Aphelenchoides hodsoni Goodey, 1935." Proc. helminth. Soc. Wash. 9 (2), 82–84.
Cralley, E.M. 1949. "White tip of rice." Phytopathology. 39:5.
European Plant Protection Agency (EPPO). 2016. Aphelenchoides besseyi. https://gd.eppo.int/taxon/APLOBE
Ferris, H. 2017. Nemaplex, the "Nematode-Plant Expert Information System." A Virtual Encyclopedia on Soil and Plant Nematodes. University of California, Davis. http://nemaplex.ucdavis.edu/
Global Biodiversity Information Facility (GBIF). 2016. GBIF Backbone Taxonomy. GBIF Secretariat. (http://www.gbif.org/species/2284354).
Kakuta, T. 1913. "Black grain disease of rice." J. PI. Prot., Tokyo. 2, 214–218. [In Japanese]
Meyer, B. 2017. "Nematodes: Getting to Know the Enemy Better." Hosta Library Reading Room. http://www.hostalibrary.org/articles/Nematodes.htm
Oliveira, C.J., Subbotin, S.A., Álvarez-Ortega, S., Desaeger, J., Brito, J.A., Xavier, K.V., Freitas, L.G., Vau, S. and Inserra, R.N. (2019). Morphological and Molecular Identification of Two Florida Populations of Foliar Nematodes (Aphelenchoides spp.) Isolated from Strawberry with the Description of Aphelenchoides pseudogoodeyi sp. n. (Nematoda: Aphelenchoididae) and Notes on Their Bionomics. Plant Disease 103: 2825-1842. https://doi.org/10.1094/PDIS-04-19-0752-RE
Subbotin, S.A., Oliveira, C.J., Álvarez-Ortega, S., Desaeger, J., Crow, W., Overstreet, C., Vau, S., Lehy, R. and Inserra, R.N. (2020). The Taxonomic Status of Aphelenchoides besseyi Christie, 1942 (Nematoda: Aphelenchoididae) Populations from Southeastern United States and Description of Aphelenchoides pseudobesseyi sp. n. Nematology pages 1-33. https://doi.org/10.1163/15685411-bja10048
Yoshi, H., and S. Yamamoto. 1950. "A rice nematode disease 'Sencha Shingane Byo'. II. Hibernation of Aphelenchoides oryzae." Journal of Faculty of Agriculture. Kyusha University. 9:223–233.