This article is intended to guide agricultural professionals in the use of cowpea cover crops for managing plant-parasitic nematodes.
Advantages
Cover crops are grown between cash crop cycles, or intercropped with cash crops to cover the ground, such as in vegetable fields, orchards, groves, and agricultural sites. If used appropriately, cover crops can improve soil structure and fertility, decrease soil erosion, provide foliage and animal feed, and suppress crop pests such as weeds, insects, nematodes, and other plant pathogens. Residues from cover crops can be incorporated as green manure to supply nutrients and improve fertility for the next crop. Using cover crops can increase on-farm crop diversity, may enhance many beneficial organisms, and could possibly even contribute to carbon sequestration. One good example of a summer cover crop is cowpea, Vigna unguiculata (L.) Walp, due to its fast establishment, abiotic and biotic stress tolerance, and biomass production (Figure 1).

Credit: E. Rios, UF/IFAS
Cowpea is a resilient crop critical for the nutrition and income of millions of families in the tropical and subtropical world. It has a rich history in the southeastern United States, where it has been grown for centuries. Cowpea is one of the most tolerant legumes to high temperature and drought, and it can be a significant contributor to sustainable cropping systems. Therefore, it is well-adapted to Florida’s hot and humid climate as well as sandy soils. For more information on cover crop production in Florida, see Wright et al. (2017). One advantage of using cowpea as a cover crop is its ability to associate with nitrogen-fixing bacteria and thus provide nitrogen for itself and the following crop (Figure 2). Besides fixing nitrogen, cowpea provides other ecosystem services such as weed and nematode suppression, but not all cowpea cultivars perform the same. Some cowpea cultivars can be damaged by several pests and diseases (Figure 3), including root-knot nematodes (Meloidogyne spp.), the key nematode pest in many cropping systems in north central Florida (Harrison et al. 2014; Dareus et al. 2021). Using susceptible cultivars as cover crops could result in severe root galling (Figure 4). When nematodes build up on cowpea or other cover crops, they can cause damage and yield loss to the next crop planted, which could be a valuable cash crop.

Credit: E. Rios, UF/IFAS

Credit: E. Rios, UF/IFAS

Credit: Mace Bauer, UF/IFAS
Host Status of Cultivars of Cowpea to Plant-Parasitic Nematodes
Fortunately, some common cultivars have resistance to one or more species of root-knot nematodes. In particular, resistance to the most common root-knot nematode species, M. incognita (southern root-knot nematode) and the invasive M. enterolobii (guava root-knot nematode) has been characterized for a number of cowpea cultivars (Table 1; Figure 5). The host status is grouped into 3 categories based on their susceptibility to the nematodes: poor, intermediate, and good hosts. Growers should avoid using good or intermediate hosts in their crop rotation if the specific nematode species are present in the field because the nematode populations will increase on these host plants. A poor host does not support reproduction of the corresponding root-knot nematode species and will be useful in managing that pest. This combination of nematode and nitrogen management could be especially useful in organic production systems where neither nematicides nor synthetic nitrogen fertilizers could be used. For example, ‘Iron Clay’ is a commonly used cowpea cultivar for summer cover cropping. It has the advantage of being resistant to M. incognita (McSorley et al. 1999; Dareus et al. 2021), although it is susceptible to M. enterolobii (Table 2; Dareus et al. 2021), an emerging pest in Florida. Harrison et al. (2014) released three cowpea germplasm lines for use as cover crops: US-1136, US-1137, and US-1138. These germplasm lines are not only softseeded, but are also resistant to certain root-knot nematodes (Table 1). The three germplasm lines were found to be as effective at suppressing weeds and root-knot nematodes as ‘Iron Clay’ (Zambon et al. 2013), and US-1136 was the only resistant cultivar to M. enterolobii in the study conducted by Dareus et al., 2021.
Cowpea cultivars have not tested as extensively against other root-knot nematode species or other plant-parasitic nematodes important in Florida (Table 2). Based on research in older cultivars, most cultivars with resistance to M. incognita are also resistant to common isolates of M. arenaria and M. javanica (Roberts et al. 2005), although many modern cultivars have not been tested against these nematodes (Table 2). Cowpea is susceptible to reniform nematode (Rotylenchulus reniformis), a major pest of cotton. In limited testing on Belonolaimus longicaudatus (sting nematode) and Paratrichodorus minor (stubby-root nematode), cowpea was detrimental for sting nematode management and was beneficial for stubby-root nematode management in Florida.
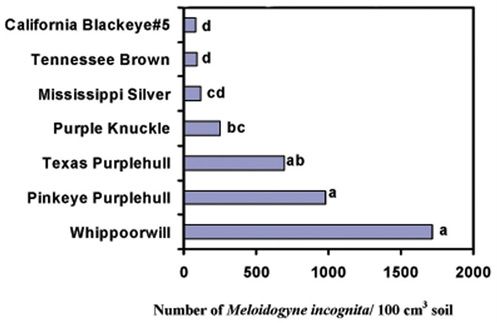
Credit: Gallaher and McSorley 1993
Challenges in Managing Nematode with Cowpea
In central Florida research, initial beneficial effects of cowpea rotation on root-knot nematode populations were lost once a susceptible vegetable crop like tomato or pepper was grown (McSorley et al. 1999). Combining cowpea cover cropping with solarization, a nematode-resistant vegetable cultivar, or other strategies may provide the organic vegetable grower with a viable means for root-knot nematode management (McSorley et al. 1999).
Determining the nematode species in a field and, if available, selecting a suitable resistant cowpea cultivar to manage the nematode(s) present is crucial for successful management. This will be more difficult if mixtures of different plant-parasitic nematode species are present in the field. Ideally, a cowpea cultivar with resistance against all nematodes in a field would be selected. Otherwise, it is critical to focus management options on the key nematode pest. If a suitable cowpea cultivar for key nematode pests in a field is not available, a different cover crop or management strategy would be needed. Additionally, responses of a root-knot nematode species to cowpea may depend on the local nematode isolates, particularly for cultivars that have an intermediate level of resistance. For example, M. javanica from Florida may reproduce better on cowpea than an isolate from Hawaii. Therefore, host status of a cultivar may vary somewhat by location. For this reason, it is always prudent to plant a new cultivar in a small area to test performance against local root-knot nematode isolates before it is widely planted. Testing of modern cultivars and breeding for resistance to Florida isolates of M. enterolobii and M. incognita is ongoing (Dareus et al. 2021). In addition, behavior (virulence) of root-knot isolates within an area might also change over time. Studies at University of California, Riverside, showed that a local population of the root-knot nematode M. incognita consisted of individuals varying in fitness. Although most of these root-knot isolates cannot reproduce on cowpea that contain the root-knot-nematode-resistant gene Rk, present in most commercial resistant cowpea cultivars, some nematode isolates can overcome the resistant gene (Roberts et al. 2005). Currently, no M. incognita that overcome root-knot-nematode resistance have been identified in Florida. To discourage development of resistance-breaking root-knot-nematode isolates, root-knot-nematode-resistant cowpea may be rotated with non-host or susceptible crops. Continuous culture of root-knot-nematode-resistant cowpea should be avoided because it will favor the resistant isolates of nematodes.
Table 1. Host status of cowpea cultivars for southern root-knot nematode (M. incognita) and guava root-knot nematode (M. eneterolobii).
Table 2. Host status of cowpea cultivars for various plant-parasitic nematodes: M. javanica (Javanese root-knot nematode), M. arenaria (peanut root-knot nematode), R. reniformis (reniform nematode), B. longicaudatus (sting nematode), and P. minor (stubby-root nematode).
References
Baujard, P., and B. Martiny. 1995. “Ecology and Pathogenicity of 4 Trichodorid Species from the Semiarid Region of West-Africa.” Nematologica 41:98–105.
Dareus, R., A. C. M. Porto, M. Bogale, P. DiGennaro, C. A. Chase, and E. F. Rios. 2021. “Resistance to Meloidogyne enterolobii and Meloidogyne incognita in Cultivated and Wild Cowpea.” HortScience 56 (4): 460–468. doi: 10.21273/HORTSCI15564-20
Fassuliotis, G. 1976. “Progress, Problems and Perspectives in Breeding Food Crops for Root-Knot Resistance” pp. 81–93 Proceedings of the Research Planning Conference on Root-Knot Nematodes, Meloidogyne spp. January 12–16, North Carolina State University, Raleigh.
Gallaher, R. N., and R. McSorley. 1993. “Population Densities of Meloidogyne incognita and Other Nematodes Following Seven Cultivars of Cowpea.” Nematropica 23:21–26.
Harris, A. R., and H. Ferris. 1991. “Interactions between Fusarium oxysporum f. sp. Racheiphilum and Meloidogyne spp. in Vigna unguculata. 2. Specificity of Different Taxa.” Plant Pathology 40:457–464.
Inserra, R. N., R. A. Dunn, and N. Vovlas. 1994. “Host Response of Ornamental Palms to Rotylenchulus reniformis.” Supplement to the Journal of Nematology 4S:737–743.
Kirkpatrick, T. L. and T. E. Morelock. 1987. “Response of Cowpea Breeding Lines and Cultivars to Meloidogyne incognita and M. arenaria.” Annals of Applied Nematology 1:46–49.
Kokalis-Burelle, N., D. M. Butler, and E. N. Rosskopf. 2013. “Evaluation of Cover Crops with Potential for Use in Anaerobic Soil Disinfestation (ASD) for Susceptibility to Three Species of Meloidogyne.” Journal of Nematology 45:272–278.
McSorley, R. 1999. “Host Suitability of Potential Cover Crops for Root-Knot Nematodes.” Supplement to the Journal of Nematology 31:619–623.
McSorley, R., and D. W. Dickson. 1995. “Effect of Tropical Rotation Crops on Meloidogyne incognita and Other Plant-Parasitic Nematodes.” Supplement to the Journal of Nematology 27:535–544.
McSorley, R., and R. N. Gallaher. 1991. “Nematode Population Changes and Forage Yields of Six Corn and Sorghum Cultivars.” Supplement to Journal of Nematology 23:673–677.
McSorley, R., and R. N. Gallaher. 1992. “Comparison of Nematode Population Densities on Six Summer Crops at Seven Sites in North Florida.” Supplement to Journal of Nematology 24:699–706.
McSorley, R., M. Ozores-Hampton, P. A. Stansly, and J. M. Conner. 1999. “Nematode Management, Soil Fertility, and Yield in Organic Vegetable Production.” Nematropica 29:205–213.
Roberts, P. A., W. C. Matthews, and J. D. Ehlers. 2005. “Root-Knot Nematode Resistant Cowpea Cover Crops in Tomato Production Systems.” Agronomy Journal 97:1626–1635.
Swanson, T. A., and S. D. Van Gundy. 1984. “Cowpea Resistant to Root Knot Caused by Meloidogyne incognita and M. javanica.” Plant Disease 68:961–964.
Waisen, P., B. S. Sipes, and K.-H. Wang. 2019. “Potential of Biofumigant Cover Crops as Open-End Trap Crops against Root-Knot and Reniform Nematodes.” Nematropica 49:254–264.
Wang, K. -H., R. J. McGovern, R. McSorley, and R. N. Gallaher. 2004. “Cowpea Cover Crop and Solarization for Managing Root-Knot and Other Plant-Parasitic Nematodes in Herb and Vegetable Crops.” Soil and crop science society of Florida proceedings. 63:99–104.
Wright, D. L., C. Mackowiak, and A. Blount. 2017. Cover crops. SS-AGR-66. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/AA217