The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Pine sawfly larvae, Neodiprion spp., are the most common defoliating insects of pine trees, Pinus spp., in Florida. Sawfly infestations can cause growth loss and mortality, especially when followed by secondary attack by bark and wood-boring beetles (Coleoptera: Buprestidae, Cerambycidae, Scolytidae). Trees of all ages are susceptible to sawfly defoliation (Barnard and Dixon 1983; Coppel and Benjamin 1965).

Credit: Arnold T. Drooz, USDA Forest Service; www.forestryimages.org
Distribution
Neodiprion spp. are indigenous to Florida. Host tree specificity and location will bear on sawfly distribution statewide.
Description
Six species are covered here so there is some variation in appearance. However, an adult female has a length of 8 to 10 mm, with narrow antennae on the head and a stout and thick-waisted body. This is unlike most Hymenopteran insects which have the thinner, wasp-like waist. The background color varies from light to dark brown, with yellow-red-white markings common. The two pairs of wings are clear to light brown with prominent veins.
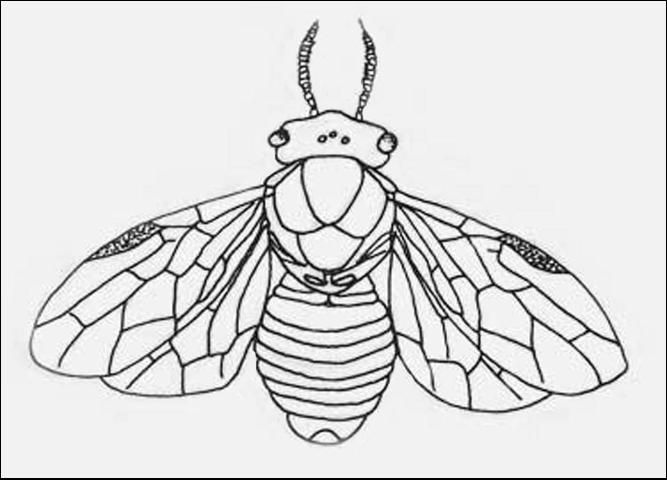

Credit: Lacy L. Hyche, Auburn University, www.forestryimages.org
Adult
The adult male has a length of 5 to 7 mm. The male has broad, feathery antennae on the head with a slender, thick-waisted body. It generally has brown to black color wings, similar to the female.

Credit: G. Keith Douce, University of Georgia, www.forestryimages.org

Credit: Gerald J. Lenhard, Louisiana State University, www.forestryimages.org
Egg
The egg is small (0.5 mm wide x 1.8 mm long), green-yellow-white color and ovoid.

Credit: Lacy L. Hyche, Auburn University, www.forestryimages.org
Larva
The length of the mature larva is 18 to 25 mm with variable coloration (see Table 1).
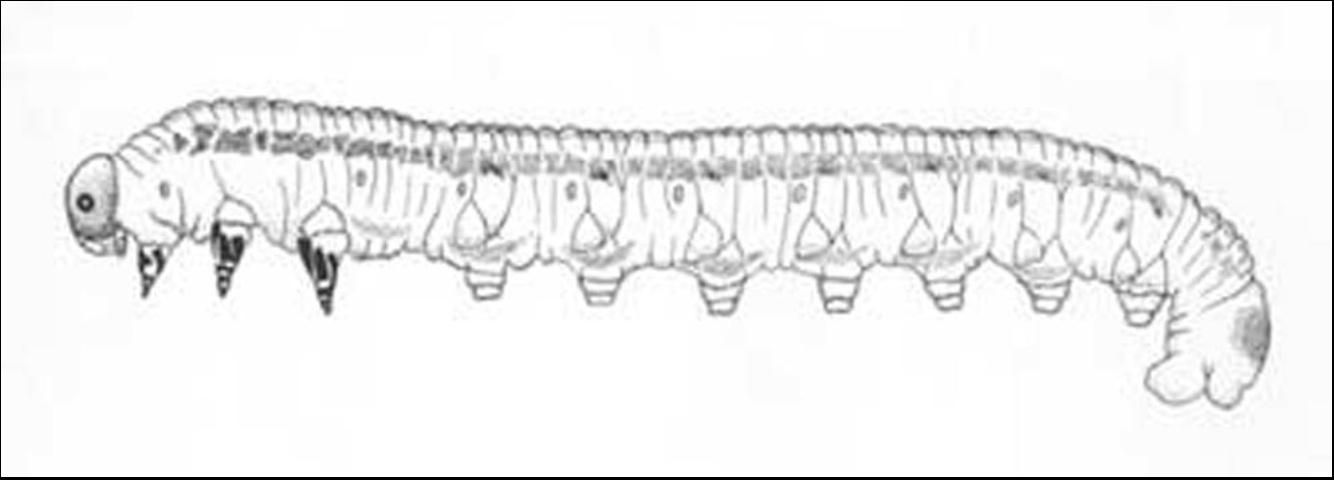
Credit: Georgia Forestry Commission
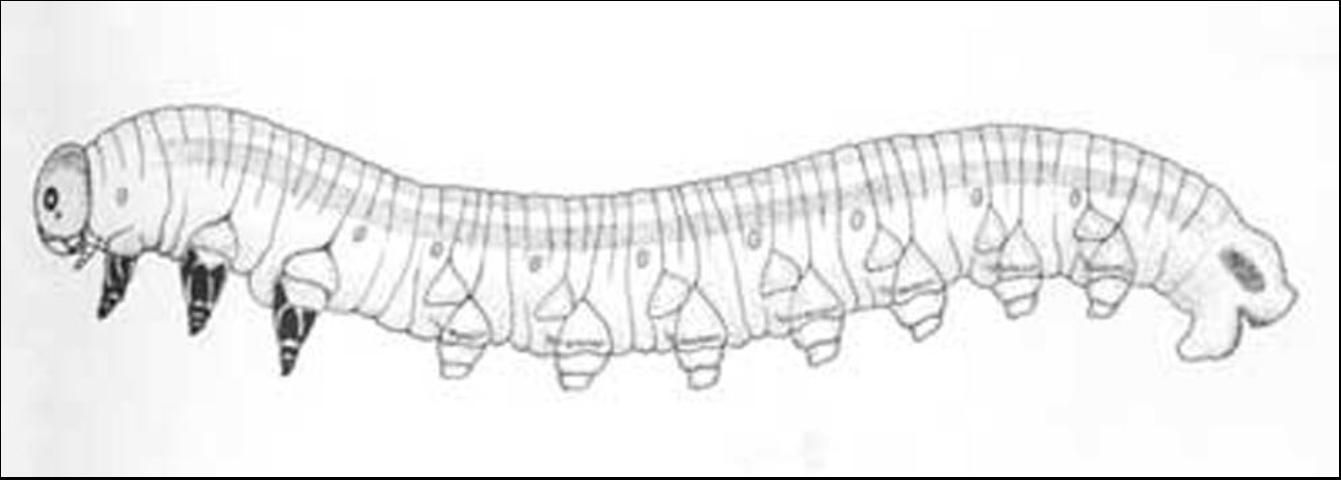

Credit: Ronald F. Billings, Texas Forest Service, www.forestryimages.org

Credit: Ronald F. Billings, Texas Forest Service, www.forestryimages.org
Pupa
The pupa length is similar to that of the adult. The cocoon is light brown to dark reddish-brown, papery, and 3.5 to 6.0 mm wide x 7.1 to 10.0 mm long (Coppel and Benjamin 1965; Thatcher 1971; Wilkinson 1965).

Credit: Jana Albers, Minnesota Department of Natural Resources, www.forestryimages.org
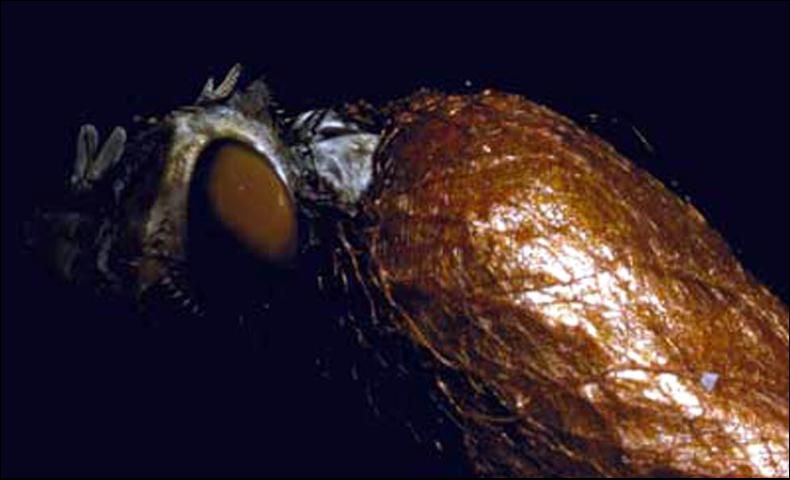
Credit: Arnold T. Drooz, USDA Forest Service, www.forestryimages.org.

Credit: Arnold T. Drooz, USDA Forest Service, www.forestryimages.org
Biology
Mature sawfly larvae spin cocoons in the duff or pine litter, mineral soil, or under bark scales. Adult sawflies emerge by removing a cap at one end of cocoon. After mating, female sawflies lay eggs in slits sawed in pine needles. Small larvae feed on outer needle tissues; larger larvae consume entire needles. Most species prefer older foliage, but all foliage is susceptible at the end of the growing season. Larval colonies may migrate from one tree to another, especially upon complete defoliation of the host tree or high feeding competition. The number of sawfly generations (one to four) varies from year to year and according to species. Larvae may diapause (a survival behavior for adverse conditions) for more than one year (Coppel and Benjamin 1965; Wilkinson 1980).

Credit: Arnold T. Drooz, USDA Forest Service, www.forestryimages.org

Credit: James McGraw, North Carolina State University, www.forestryimages.org
Hosts
All southern pines, Pinus spp., are susceptible to sawfly infestation.

Credit: Arnold T. Drooz, USDA Forest Service, www.forestryimages.org

Credit: G. Keith Douce, University of Georgia, www.forestryimages.org

Credit: G. Keith Douce, University of Georgia, www.forestryimages.org

Credit: Caleb L. Morris, Virginia Department of Forestry, www.forestryimages.org
Survey and Detection
Early damage is evidenced by reddish-brown straw like remains of needles that are incompletely consumed by young larvae; older larvae leave only short stubs. Partially defoliated branches often have a "bottle brush" appearance. Sawfly colonies may consist of a few to over a hundred individuals. Upon disturbance, larvae may drop from branches or assume a U-bend by raising head and abdomen. An oral exudate, which can paralyze insect parasitoids and repel predators, often accompanies such displays (Barnard and Dixon 1983; Coppel and Benjamin 1965).

Credit: G. Keith Douce, University of Georgia, www.forestryimages.org
Management
Suppression of sawfly populations by insecticides is usually successful. However, consideration should be given to conserving natural enemies (small mammals, birds, insects) through minimal insecticide use and preservation of cypress-hardwood pond stands around pine plantations. The appearance of numerous dead larvae hanging from needles, i.e., virus-infected, usually signals the collapse of a sawfly outbreak. Sawfly outbreaks are cyclical—an eight-to-10-year interval is common. A fully stocked stand and promotion of early crown closure minimizes risk of sawfly damage in pine plantations (Wilkinson 1980).
Selected References
Atwood CE. 1961. Present status of the sawfly family Diprionidae (Hymenoptera) in Ontario. Proceedings of the Entomological Society of Ontario 91: 205–215.
Barnard EL, Dixon WN. 1983. Insects and diseases; important problems of Florida's forest and shade tree resources. Florida Department of Agriculture and Consumer Services, Division of Forestry Bulletin No. 196A. 123 pp.
Coppel HC, Benjamin DM. 1965. Bionomics of the Nearctic pine-feeding diprionids. Annual Review of Entomology 10: 69–96.
Thatcher RC. 1971. Spine sawfly, Neodiprion excitans Roh. U.S. Department of Agriculture. Forest Servive Pest Leaflet 105. 4 pp.
Wilkinson RC. 1965. Neodiprion merkeli from south Florida. Florida Entomologist 48: 271.
Wilkinson RC. 1980. Pine sawflies in Florida. In Forest pest management 12th spring symposium for the Florida section of the Society of American Foresters, 3–4 June 1980. University of Florida, Institute of Food and Agricultural Sciences Resources Report 7: 53–55.
Wilson LF. 1970. The red-headed pine sawfly. U.S. Department of Agriculture Forest Service Forest Pest Leaflet 14. 6 pp.