Introduction
Chinese privet or hedge privet, Ligustrum sinense (Lour.) (Oleaceae), is a semi-deciduous shrub or small tree of Asiatic origin that is commercially available from the nursery industry as a landscape ornamental (Broschat and Meerow 1991). This woody shrub is frequently used as a hedge or border plant in Florida because it adds color and design to landscapes, especially in its variegated form (Watkins and Sheehan 1975; Whitcomb 1975; Dehgan 1998).
A seed-attacking weevil was found attacking Chinese privet in Tallahassee, Leon County, Florida (Cuda and Zeller 1998). The insect was discovered in a sample of several hundred seeds collected for germination studies. Six weevil adults that emerged from the infested seed sample were later identified (by M. C. Thomas) as the ligustrum weevil, Ochyromera ligustri Warner. The remaining seed stock from Tallahassee was dissected and inspected for the presence of the insect. Weevil larvae were recovered from 89 of the 358 seeds examined. Overall, 24.9% of the Chinese privet seeds collected were infested with weevil larvae. Voucher specimens of the larval and adult stages of the ligustrum weevil were deposited in the Florida State Collection of Arthropods, Division of Plant Industry, Florida Department of Agriculture and Consumer Services, Gainesville.

Credit: Jon Hart, bugguide.net
Distribution
The ligustrum weevil has been reported from Florida, Georgia, North and South Carolina, and Virginia (O'Brien and Wibmer 1982; Johnson and Lyon 1991). In Florida, this insect occurs in the northern part of the state where established populations have been documented in Jefferson and Leon Counties (O'Brien and Wibmer 1982; Cuda and Zeller 1998, 1999, 2000; Bloem et al. 2002).
Description
Weevils of the genus Ochyromera Pascoe are small- to medium-sized insects characterized by having the profemora (or front legs) armed with a large unserrated triangular tooth and the antennae consisting of seven segments in the funicle (or apical portion) (Warner 1961).
Egg
The egg stage of the ligustrum weevil has not been described.
Larva
Although the larval stage has not been formally described, the larvae of the ligustrum weevil are typical of most weevil larvae in that they are soft bodied, somewhat C-shaped, cream colored in appearance, and without legs. The distinctive urogomphus (or terminal cuticular process) visible on the apical abdominal segment of the ligustrum weevil larva is a unique character. Larvae of the Curculionidae normally are differentiated from related families by the complete absence of terminal abdominal spines (Anderson 1991).
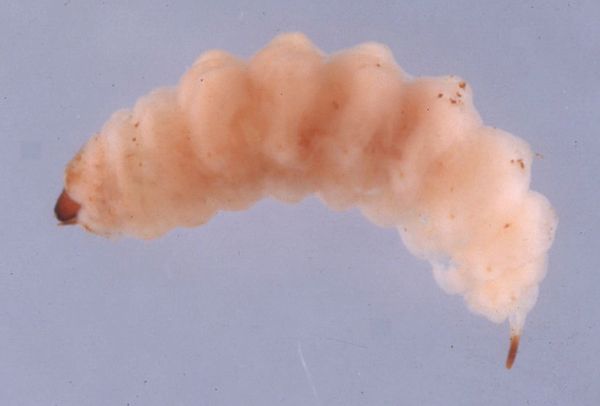
Credit: Jerry Butler, UF/IFAS
Pupa
The pupal stage of the ligustrum weevil has not been described.
Adult
The adult is shiny brown with golden yellow hair-like scales. The prothorax is wider than long with a definite median stripe and somewhat obscure lateral stripes when viewed from above. Females are larger than males (3.9 mm versus 3.7 mm) and have a longer snout (1.4 mm versus 1.1 mm).

Credit: J. C. Jones, bugguide.net
Life Cycle
The biology of the ligustrum weevil on Japanese privet, L. japonicum Thunb., was reviewed by Johnson and Lyon (1991). The adults feed on the foliage by making small perforations (3 to 4 mm long) in the interior of the lamina (or leaf blade) and occasionally along the leaf margins. The weevil is easily alarmed and will drop to the ground when disturbed during feeding or while resting on a host plant. In North Carolina, adults are active from late June to early July. Females deposit their eggs in the seed or in the mature fleshy fruits. Larvae hatch within a few weeks. The young larvae feed inside the fruits during the fall and winter months. Full-grown larvae and pupae appear in late April and early May during the following year. The larvae destroy the seeds as they complete their development to the adult stage. New adults emerge from the seeds (one weevil per seed) in mid-May, and there is only one generation per year.
Hosts
The ligustrum weevil was first discovered in 1959 on Japanese privet in Wake County, North Carolina (Warner 1961; Wray 1961), and is believed to have immigrated from the Orient in nursery stock imported into the United States. Although it prefers Japanese privet, the ligustrum weevil also has been collected from wax-leaf ligustrum, L. lucidum Ait.; common privet, L. amurense Carr.; lilac, Syringa spp. and grape, Vitis spp., in North Carolina (Warner 1961; Wray 1961). It is not clear from the literature whether lilacs or grapes are true host plants capable of supporting complete development of the weevil. However, Kojima et al. (1998) reported that adults have been collected on Japanese privet, wax-leaf ligustrum, common privet, and lilac, but larvae were found only in Japanese privet. Japanese privet is documented as naturalized in Leon, Wakulla, Taylor, and Volusia counties (Wunderlin et al. 2017). It is not surprising that the weevil has adapted to Chinese privet in this region of north Florida where the two species of Ligustrum have sympatric (or overlapping) distributions.
Economic Importance
Depending on the location, the ligustrum weevil may be considered harmful or helpful. Chinese privet is still regarded as a desirable plant for landscaping in many areas of the southeastern United States because of its attractive flowers, dark green or variegated foliage, resistance to most pests and diseases, and general hardiness. Where it is being cultivated as an ornamental or for erosion control, feeding damage by the adult weevil destroys the buds of Chinese privet, causing bunchy growth and a tattered appearance (Baker 1980). To minimize the damage, the ligustrum weevil can be controlled mechanically by shearing off the flowers and fruits (Baker 1980).
However, the ligustrum weevil may be considered a beneficial species in natural areas that have been invaded by Chinese privet. Following its introduction into the United States in 1852 (Dirr 1983), Chinese privet eventually escaped cultivation, and by 1932 had become naturalized across the southeastern United States (Small 1933). Chinese privet is widely naturalized from Massachusetts to Texas and north to Missouri (USDA, NRCS 2014). It is considered an invasive weed in the southeastern Unites States (Dirr 1983; Nelson 1996; Miller 2003), including north Florida, Alabama, Georgia (Godfrey 1988), Mississippi (Goddard 1992), and Tennessee (Faulkner et al. 1989). Songbirds and bobwhite quail are primarily responsible for spreading the plant by ingesting the fruits and dispersing the seeds (MacRae 1980). In some cases, Chinese privet forms dense thickets that displace more desirable native vegetation (Faulkner et al. 1989). According to Goddard (1992), dense stands of this weedy shrub also may harbor populations of the blacklegged or deer tick Ixodes scapularis Say, a suspected vector of Lyme disease in the southern United States (Oliver 1989).
The Florida Exotic Pest Plant Council currently lists Chinese privet as a Category I invasive species, because there is documented evidence it causes severe ecological damage by disrupting native plant communities (FLEPPC 2003). Because Chinese privet is considered a prohibited plant in Florida, UF/IFAS faculty cannot recommend it for landscaping purposes.(UF/IFAS Assessment 2017).
By attacking the seeds of Chinese privet, the ligustrum weevil may be important for adventive biological control of this invasive weed. As an immigrant natural enemy of Ligustrum spp., the ligustrum weevil may be capable of reducing the spread of Chinese privet into new areas and/or the densities of existing stands. However, field and laboratory studies will be required to determine the extent to which this insect is capable of controlling the growth and spread of Chinese privet populations in Florida's natural areas.
Selected References
Anderson DM. 1991. Curculionidae (Broad Sense) (Curculionoidea), pp. 594-612. In Stehr FW (ed.). Immature Insects, Volume 2. Kendall/Hunt Publishing Co., Dubuque. 975 pp.
Baker JR (ed). 1980. Insect and related pests of shrubs: Some important, common and potential pests in the southeastern United States. North Carolina Agricultural Extension Service, AG-189. Raleigh, North Carolina.
Bloem S, Mizell III RF, O'Brien CW. 2002. Old traps for new weevils: New records for curculionids (Coleoptera: Curculionidae), Brentids (Coleoptera: Brentidae) and Anthribids (Coleoptera: Anthtribidae) from Jefferson Co., Florida. Florida Entomologist 85: 632–644.
Broschat TK, Meerow AW. 1991. Betrock's reference guide to Florida landscape plants, 4th printing 1999. Betrock Information Systems, Inc., Hollywood, FL. 428 pp.
Cuda JP, Zeller MC. 1998. First record of Ochyromera ligustri (Coleoptera: Curculionidae) from Chinese privet in Florida. Florida Entomologist 81: 582–584.
Cuda JP, Zeller MC. 1999. Ochyromera ligustri (Coleoptera: Curculionidae), an immigrant natural enemy of Chinese privet Ligustrum sinense Lour. (Oleaceae) in Florida, pp. 357-362. In Jones DT, Gamble BW (eds.), Proceedings of the 1998 Joint Symposium of the Florida Exotic Pest Plant council and the Florida Native Plant Society, Palm Beach Gardens, 3–7 June 1998.
Cuda JP, Zeller MC. 2000. Chinese privet, Ligustrum sinense: Prospects for classical biological control in the southeastern United States. Wildland Weeds 3: 17–19.
Dehgan B. 1998. Landscape plants for subtropical climates. University of Florida Press, Gainesville. 638 pp.
Dirr MA. 1983. Manual of woody landscape plants, 3rd ed. Stipes Publishing Co., Champaign, IL. 536 pp.
Faulkner JL, Clebsch EEC, Sanders WL. 1989. Use of prescribed burning for managing natural and historic resources in Chickamauga and Chattanooga National Military Park, USA. Environmental Management 13: 603–612.
FLEPPC. (2017). Florida Exotic Pest Plant Council's 2017 List of Invasive plant species. (07 February 2022). https://www.wbdg.org/FFC/AF/AFIFS/FLEPPC_Invasive_Plant_List_2017.pdf
Goddard J. 1992. Ecological studies of adult Ixodes scapularis in central Mississippi: questing activity in relation to time of year, vegetation type, and meteorologic conditions. Journal of Medical Entomology 29: 501–506.
Godfrey R K. 1988. Trees, shrubs and woody vines of northern Florida and adjacent Georgia and Alabama. University of Georgia Press, Athens. 734 pp.
Johnson WT, Lyon HH. 1991. Insects that feed on trees and shrubs, 2nd ed., revised. Cornell University Press, Ithaca. 556 pp.
Kojima H, Morimoto K, Horikawa M. 1998. Two new species of the genus Ochyromera (Coleoptera: Curculionidae) from Japan. Esakia 38: 113–122.
MacCrae WA. 1980. Unusual bobwhite foods on abandoned Piedmont farmlands, Georgia, USA. Georgia Journal of Science 38: 49–54.
Miller, J.H. 2003. Nonnative invasive plants of southern forests: a field guide for identification and control. USDA Forest Service, Southern Research Station General Technical Report SRS-62.
Nelson G. 1996. The shrubs and woody vines of Florida. Pineapple Press, Sarasota. 391 pp.
O' Brien CW, Wibmer G J. 1982. Annotated checklist of the weevils (Curculionidae sensu lato) of North America, Central America, and West Indies (Coleoptera: Curculionoidea). Memoirs of the American Entomological Institute 34: 116.
Oliver Jr JH. 1989. Lyme disease: tick vectors, distribution and reservoir hosts. Journal of the Medical Association of Georgia 78: 3–6.
Small JK. 1933. Manual of the southeastern flora, part one and two. University of North Carolina Press, Chapel Hill. 1554 pp. (facsimile reprint 1972, Hafner Publishing, New York.).
UF/IFAS Assessment. (2017). UF/IFAS assessment of non-native plants in Florida's natural areas. ( 16 June 2017). https://assessment.ifas.ufl.edu/assessments/ligustrum-sinense/
USDA, NRCS. 2014. The PLANTS Database (http://plants.usda.gov, 3 June 2014). National Plant Data Team, Greensboro, NC 27401-4901 USA.
Warner RE. 1961. The genus Ochyromera new to the western hemisphere, with a new species and additions to the Junk-Schenkling Coleopterorum Catalogus (Curculionidae: Prionomerinae, Endaeini). Coleopterists' Bulletin 15: 121–124.
Watkins JV, Sheehan TJ. 1975. Florida landscape plants. University Press, Gainesville, 368 pp.
Whitcomb CE. 1975. Know it and grow it. Oklahoma State University Press, Stillwater, 500 pp.
Wray DL. 1961. Biology and life history of the ligustrum weevil (Curculionidae). Coleopterists'Bulletin 15: 119–120.
Wunderlin RP, Hansen BF, Franck AR, Essig FB. 2017. Atlas of Florida Plants (http://florida.plantatlas.usf.edu/). [. Landry SM, Campbell KN (application development), USF Water Institute.] Institute for Systematic Botany, University of South Florida, Tampa (26 June 2017).