The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Culex (Melanoconion) iolambdis (Dyar 1918) is a small, dark mosquito that tends to feed on reptiles and amphibians. It is found in the southeastern United States and many countries in Central America and South America. It has not been identified as a species of medical importance as it has not been shown to vector pathogens like some other Culex species.
Distribution
Culex iolambdis occurs from Mexico south to Colombia and Ecuador and east to Florida. Culex iolambdis has been reported in collections from Cuba, Jamaica, Puerto Rico, and the Cayman Islands.
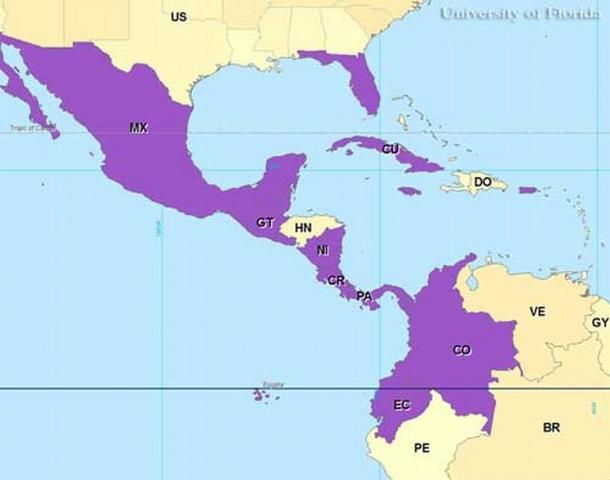
Credit: Gregg Ross, University of Florida
In the US, Culex iolambdis has been reported in collections from 10 counties in Florida: Charlotte, Collier, Dade, Indian River, Lee, Manatee, Martin, Monroe, Polk, and St. Lucie.
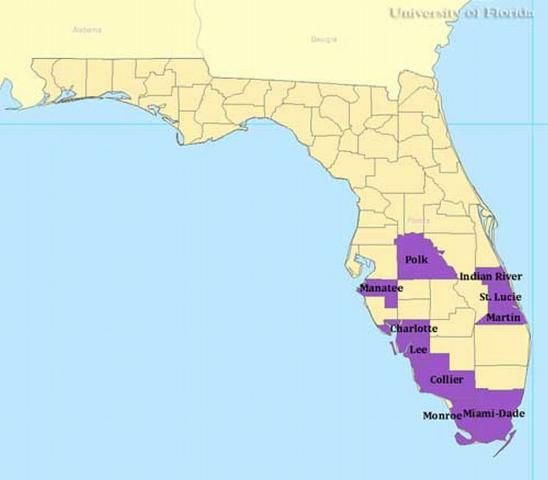
Credit: Gregg Ross, University of Florida
Description
Adults
Species in the subgenus Melanoconion are noted for the wide scales on their wings, flat scales on the posterior of the head, and their small size. The wing length of adult Cx. iolambdis varies from 2.0 mm to 2.18 mm (Knight and Haeger 1971, Belkin et al. 1970). The mosquito is dark brown (Pratt and Seabrook 1952) to black and the abdomen can appear to be bluish-black. The scales on the posterior of the head have a metallic reflection (Carpenter and LaCasse 1955). The proboscis is long, dark, and has a bulb-like tip (Carpenter and LaCasse 1955). The dorsal surface of the thorax is covered in brownish-black to shiny black scales, and dark brown setae (Knight and Haeger 1971). At the base of the last few segments of the abdomen there are a few white scales (Pratt and Seabrook 1952). The legs have a bronze reflection with pale tips (Carpenter and LaCasse 1955).

Credit: C. Roxanne Connelly, UF/IFAS

Credit: James M. Newman, UF/IFAS
It is often difficult to distinguish between females in the subgenus Melanoconion, and Williams and Savage (2009) found that an internal structure used for feeding, the cibarial armature, was a useful character for identifying females in the Melanoconion subgenus.
Eggs
The eggs of Cx. iolambdis are laid in oval rafts that may contain 100 or more individual eggs. Generally, the eggs will hatch into 1st instar larvae within two hours of being deposited.

Credit: James M. Newman, UF/IFAS
Larvae
A common characteristic of species in the subgenus Melanoconion is that the larval head is wider than long and the thorax is the same size as the head (Foote 1952). The abdomen has eight segments, the siphon, and the saddle. The first two abdominal segments are two times as wide as they are long. The remaining segments taper to the siphon and the saddle (Foote 1952). In Cx. iolambdis larvae the antennae are almost as long as the head (Carpenter and LaCasse 1955). The siphon is five times as long as it is wide, with a black band one-third of the way down the siphon (Pratt and Seabrook 1952), and a row of pectin spines occurs from the base of the siphon for approximately one-third the length of the siphon (Darsie and Ward 2005, Pratt and Seabrook 1952). Beyond the row of pectin spines, five pairs of long-barbed tufts of setae occur. There is a dark scleritized plate on segment X (Pratt and Seabrook 1952), and at the end of the saddle there are four anal papillae that are shorter than the saddle plate (Pratt and Seabrook 1952).

Credit: James M. Newman, UF/IFAS
Pupae
As with all mosquito species, Cx. iolambdis pupae have two body parts, the cephalothorax and the abdomen. The cephalothorax and the abdomen are light tan with trumpets that are used for breathing (Darsie 2002). The paddles, at the apex of the abdomen, are light tan and oval in shape (Darsie 2002).

Credit: James M. Newman, UF/IFAS
Biology
Adult Culex iolambdis have been identified from Centers for Disease Control Light Trap collections (unpublished data, Connelly) and resting shelter aspirations (Blosser et al. 2016) in Indian River County, Florida, during all months of the year. This species is known to feed on a very wide range of host animals, including birds, mammals, reptiles and amphibians (Edman 1979; Blosser et al. 2016). Blood from recently fed Cx. iolambdis females from Indian River County, Florida, were analyzed to determine the bloodmeal sources and demonstrated that the most commonly bitten hosts were Green heron, American alligator, green anole, Raccoon and Souther leopard frog (Blosser et al. 2016). Other hosts included eastern cottontail, Cuban tree frog, and American oystercatcher (unpublished data, Connelly).
Culex iolambdis populations are primarily associated with mangroves in Florida (Blosser et al. 2016) and probably throughout the American tropics (Pratt and Seabrook 1952).

Credit: Erik M. Blosser, UF/IFAS

Credit: Erik M. Blosser, UF/IFAS
Larvae have been found in crab holes, coastal rock holes, mangrove swamps, and brackish swamps (Pratt and Seabrook 1952, Darsie 2002, Belkin et al. 1970).
Medical Importance
Venezuelan equine encephalitis virus has been found in Cx. iolambdis. It is not known if this species can transmit the pathogen to other hosts (Scherer et al. 1971).
Management
Culex iolambdis is not considered a mosquito species of medical importance and does not usually occur in substantial numbers to be considered a major mosquito pest species. As a result it is not usually targeted for mosquito control and there are not any methods currently recommended for controlling this species.
Selected References
Belkin JN, Heinemann SJ, Page WA. 1970. Mosquito Studies (Diptera, Culicidae) XXI. The Culicidae of Jamaica. Contributions of the American Entomological Institute 6(1).
Blosser, EM, Stenn, T, Acevedo, C, and Burkett-Cadena, ND. 2016. Host use and seasonality of Culex (Melanoconion) iolambdis (Diptera: Culicidae) from eastern Florida, USA. Acta tropica, 164, pp. 352–359.
Carpenter SJ, LaCasse WJ. 1955. Mosquitoes of North America (North of Mexico). University of California Press, Berkeley and Los Angeles, CA. pp 360.
Darsie Jr, RF. 2002. Redescription of the pupa of Culex (Melanoconion) iolambdis Dyar. Journal of the American Mosquito Control Association 18: 277–279.
Darsie Jr, RF, and Ward RA. 2005. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. University Press of Florida, Gainesville, FL. pp. 400.
Edman, JD. 1979. Host-feeding patterns of Florida mosquitoes (Diptera: Culicidae). Journal of Medical Entomology 15: 521–525.
Foote RH. 1952. The larval morphology and chaetotaxy of the Culex subgenus Melanoconion. Annals of the Entomological Society of America 45: 445–472.
Knight JW, Haeger JS. 1971. Key to adults of the Culex subgenera Melanoconion and Mochlostyrax of eastern North America. Journal of Medical Entomology 8: 551–555.
Mattingly PF. 1976. Mosquito eggs XXVIII: Culex subgenera Melanoconion and Mochlostyrax. Mosquito Systematics 8: 223–231.
Pecor JE, Mallampalli VL, Harbach RE, Payton EL. 1992. Catalog and illustrated review of the subgenus Melanoconion of Culex (Diptera: Culicidae). Contributions of the American Entomological Institute. 27.
Porter JE. 1967. A check list of the mosquitoes of the Greater Antilles and the Bahama and Virgin Islands. Mosquito News 27: 35-41.
Pratt HD, and Seabrook EL. 1952. The occurrence of Culex iolambdis Dyar in Florida and Puerto Rico, with a description of the larva. Proceedings of the Entomological Society of Washington 54: 27–32.
Scherer WF, Dickerman RW, La Fiandra RP, Wong Chia C, Terrian J. 1971. Infections of wild mammals: ecologic studies of Venezuelan encephalitis virus in southeastern Mexico. American Journal of Tropical Medicine and Hygiene 20: 980–988.
Williams MR, Savage HM. 2009. Identification of Culex (Melanoconion) species of the United States using female cibarial armature (Diptera: Culicidae). Journal of Medical Entomology 46: 745–752.
Wirth WW. 1945. The occurrence of Culex (Melanoconion) elevator Dyar and Knab in Florida, with keys to the Melanoconions of the United States (Diptera, Culicidae). Proceedings of the Entomological Society of Washington 47: 201–210.