During the past few decades, the economic importance of the tropical vegetable industry in south Florida has risen steadily. In Miami-Dade County, substantial commercial acreage is planted to three tropical vegetables: boniato (red-skinned, white-fleshed sweet potato), malanga (cocoyam, yautia), and cassava (yuca, manioc). Recent data indicate that these three crops account for 17% of the total gross farm-gate value of all the vegetables grown in the county.
Tropical vegetables are affected by several plant diseases incited by pathogenic microorganisms such as bacteria, fungi, and viruses. These disorders are often not recognized as plant diseases, and their impact on crop production is not adequately appreciated. This fact sheet describes the symptoms of several diseases commonly observed on Florida tropical vegetables and provides recommendations for control. Since few pesticides are registered by the U. S. Environmental Protection Agency for use on tropical vegetables and the reaction of pesticides on the crops are for the most part unknown, consult your local UF/IFAS Extension office or the Plant Disease Management Guide (https://edis.ifas.ufl.edu/TOPIC_BOOK_Plant_Disease_Management_Guide) for possible pesticide recommendations.
Cassava Bacterial Blight
Bacterial blight, caused by the bacterium Xanthomonas campestris pv. manihotis, has perhaps caused more damage globally than any other disease of cassava. It occurs nearly every year in Miami-Dade County, although severe outbreaks are sporadic and are usually confined to a limited number of plantings. Sufficient technology exists to control bacterial blight through the integration of several recommended measures. This disease could be reduced to one of minor importance if these steps were taken on an industry-wide scale.
Bacterial blight is characterized by a wide range of symptoms, including leaf spotting and blight, wilt, die back of twigs and stems, and discoloration of the vascular (water-conducting) tissue of infected petioles, twigs, and stems. Leaf spots begin as distinct, water-soaked, angular lesions on the lower leaf surfaces (Figure 1). Spots enlarge and can coalesce, forming brown, dried areas visible on both upper and lower leaf surfaces (Figure 2). In some cultivars, distinct yellow halos surround these brown leaf lesions.


Leaves may become completely blighted and wither. At this point, bacteria proceed into vascular tissues of the petiole and woody stems. A sticky, yellowish gum may exude from cracks in infected petioles and stems. Discolored vascular strands can often be observed if petioles and stems are cut open. Older stems of infected plants remain symptom free, although vascular tissues will sometimes show discoloration when stems are cut.
The bacterial blight pathogen is usually introduced into a new planting by infected stem cuttings. Once introduced, splashing rain is probably the major mode of spread. The organism is also readily spread mechanically on the hands and clothing of workers. Contaminated farm tools can account for rapid and widespread movement of the pathogen within fields. Since planting stock is often selected and prepared at harvest time, new crops can be infected from symptomless stems carrying the pathogen or by stem sections contaminated by infected cutting tools.
Observations at the UF/IFAS Tropical Research and Education Center in Homestead, Florida, suggest that insects (e.g., banded cucumber beetle) may play a role in the movement of X. campestris pv. manihotis. The bacterium survives poorly in the soil and its host range is thought to be limited to cassava. Disease development is favored by widely fluctuating day/night temperatures (15°C–30°C/57°F–86°F). This disease is most prevalent during the rainy season.
This potentially devastating disease can be managed quite well through the adoption of a series of integrated control measures. Crop rotation is an important control. Infected crop debris should be promptly incorporated into the soil where the bacteria die rapidly: six months is sufficient to prevent pathogen carry-over. Weeds should be controlled because X. campestris pv. manihotis can survive on the leaves and in the rootzone of some weeds.
Clean planting stock is required. If only pathogen-free stock is introduced into new plantings, disease damage can be greatly reduced. Infected stem cuttings can be rendered pathogen-free if rooted from stem tips. Details of the appropriate procedures for preparation of planting stock can be obtained by contacting your local UF/IFAS Extension office.
Dasheen Mosaic of Malanga
Dasheen mosaic is a widespread disease of malanga caused by dasheen mosaic virus (DMV). DMV is common in Florida, also affecting taro (Colocasia) and many ornamental aroid species. It occurs in nearly-all malanga plantings, affecting as many as 90% of the plants in some fields. The disease is thought to reduce yield, but no systematic studies have ever been done to actually quantify losses from dasheen mosaic.
As with many viral diseases, symptoms of DMV infection are quite subtle and can be overlooked by the casual observer. Often, the only evidence of infection is a mild, light and dark mottled appearance of the foliage (Figure 3). In some cases, more striking symptoms could appear. Feather-like patterns of light-yellow tissue may develop along veins (Figure 4). Severely infected plants may be noticeably slower in growth than surrounding plants in the field.


DMV is transmitted by several species of aphids. Aphid-mediated field spread can be very rapid. The virus particles become attached to the stylet (feeding probe) of the aphid when it feeds on an infected plant. Within a short time (probably less than one hour), the virus must then be transferred to another host if infection is to take place.
The virus is carried in infected planting stock. Since malanga is propagated exclusively by means of vegetative cuttings of corms and cormels, the virus is continually introduced into new plantings.
Techniques to provide high levels of DMV control are known but are at present cost-prohibitive for the edible aroids. The virus can be eliminated from planting stock through tissue culture, a propagation method now widely used in the ornamental aroid industry. Currently costs are sufficiently high to prohibit its use by malanga growers. It is believed that tissue culture and even more sophisticated techniques may lower costs enough in the future to make possible virus-free propagation of malanga.
Insecticidal control of aphids is of little value in DMV control. Aphids can transmit the virus before they are killed by insecticides.
Malanga Bacterial Leaf Spot
Bacterial leaf spot of malanga, caused by Xanthomonas campestris pv. dieffenbachiae, was described and characterized in the United States in the mid-1980s. It is the most commonly encountered disease problem in Miami-Dade County malanga fields, with nearly 100% of the plants showing symptoms during the rainy season.
Disease symptoms begin as small, water-soaked spots on the lower surface of leaves. Spots enlarge and substantial areas of brown, dead tissue surrounded by yellow halos develop on upper leaf surfaces (Figure 5). Pronounced water-soaking continues on the lower leaf surface, sometimes with a cream to light yellow bacterial exudate (sticky ooze) in the center of the water-soaking area (Figure 6). Lesions along the margins of leaves are often seen (Figure 7). Apparently, infection occurs readily through hydathodes (water pores) on leaf margins.



The pathogen can invade vascular (water conducting) tissue of leaves, causing water-soaked streaks along major veins (Figure 8). Once inside the vascular tissue, bacteria may be able to reach the corms and cormels, thus contaminating potential planting material.
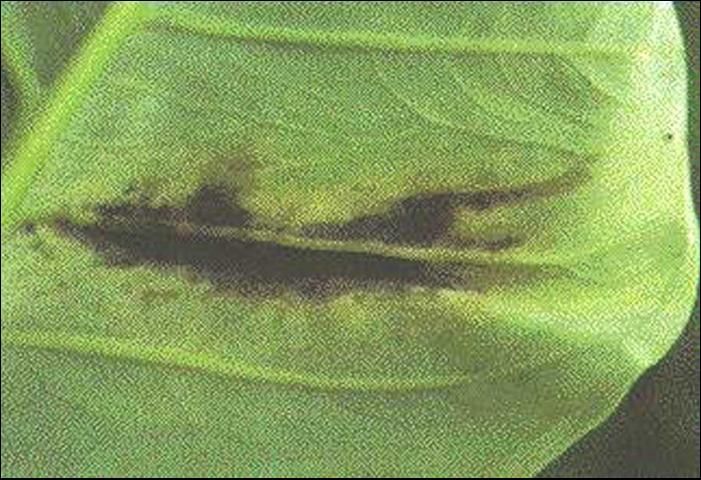
The main mode of bacterial movement is probably by splashing rain. Disease development is favored by high temperatures in the range of 29°C–32°C (85°F–90°F). Thus, the problem is most evident in the summer months. Entry of the bacterium into the plant is facilitated by wounding. The relationship between bacterial leaf spot severity and yield is unknown. Therefore, it is difficult to comment on the potential usefulness of disease control measures. Growers should stay out of fields when plants are wet, as X. campestris pv. dieffenbachiae is readily transmitted whenever workers, tools, or farm machinery contact infected, sodden plant material. Since planting stock likely is contaminated with the pathogen, the same tissue culture method that someday may be used for dasheen mosaic virus control may also help reduce bacterial spot.
Acknowledgment
The authors gratefully acknowledge the assistance of Carl Campbell and Jorge Peña in the preparation of this fact sheet.