Uterine diseases are highly prevalent in high-producing dairy cows. Metritis affects about 20% of lactating dairy cows, with the incidence ranging from 8% to >40% at some farms (Curtis et al. 1985; Galvão et al. 2009a; Goshen and Shpigel 2006; Hammon et al. 2006; Huzzey et al. 2007; Markusfeld 1984). Clinical endometritis also affects about 20% of lactating dairy cows, with the prevalence ranging from 5% to >30% in some herds (Galvão et al. 2009a; LeBlanc et al. 2002; McDougall et al. 2007). Subclinical endometritis is the most prevalent of all uterine diseases; it affects ~40%–50% of lactating dairy cows, with the prevalence ranging from 30% to >70% in some herds (Galvão et al. 2009a; Galvão et al. 2009b; Gilbert et al. 2005; Hammon et al. 2006; Kasimanickam et al. 2004; Kasimanickam et al. 2005).
Traditionally, risk factors associated with metritis include primiparity, dystocia, twins, retained placenta (RP), stillbirth, abortion, prolapsed uterus, and ketosis (Erb et al. 1981a; Erb et al. 1981b; Dohoo and Martin 1984; Markusfeld 1984; Curtis et al. 1985; Markusfeld 1985; Markusfeld 1987; Gröhn et al. 1990; Correa, Erb, and Scarlett 1993; Kaneene and Miller 1995; Goshen and Shpigel 2006; Dubuc et al. 2010). Risk factors for endometritis include dystocia, twins, RP, stillbirth, abortion, metritis, problems with vulval conformation, male offspring, and ketosis (Gröhn et al. 1990; Galvão et al. 2009a; Dubuc et al. 2010; Potter et al. 2010; Cheong et al. 2011). While metritis is more prevalent in primiparous cows (Markusfeld 1985; Markusfeld 1987; Goshen and Shpigel 2006), incidence of endometritis has been found to increase, to decrease, or to be conditional with the level of milk yield in primiparous compared to multiparous cows (Galvão et al. 2009a; Potter et al. 2010; Cheong et al. 2011). Interestingly, multiparous cows have increased bacterial contamination ~50 days after calving compared to primiparous cows (Galvão et al. 2009a). Milk production has a detrimental effect on leukocyte function (Kimura, Goff, and Kehrli 1999; Nonnecke et al. 2003); therefore, leukocytes from multiparous cows are expected to be more severely affected because of greater milk yields. In fact, phagocytic activity of neutrophils in older cows is more markedly reduced after calving compared to younger cows (Kehrli, Nonnecke, and Roth 1989; Gilbert et al. 1993). Therefore, increased levels of pro-inflammatory cytokine production in the uterine endometrium might help to prevent metritis; however, because multiparous cows have greater demands for milk yield, they might be less able to clear an infection completely and, therefore, might be more likely to have endometritis. Another important factor that might be involved in the susceptibility to metritis is the circulating levels of immunoglobulins. Immunoglobulins work as opsonins, which greatly enhance phagocytic capacity. Primiparous cows have lower immunoglobulin content in colostrums, which indicates lower circulating immunoglobulin levels (Muller and Ellinger 1981); therefore, phagocytosis might not be optimal in early lactation in primiparous cows.
Recent studies have focused on the effect of dry matter intake, indicators of energy balance such as nonesterified fatty acids (NEFA) and betahydroxybutyrate (BHBA), haptoglobin, glycogen stores in neutrophils, and calcium on uterine disease (Hammon et al. 2006; Huzzey et al. 2007; Duffield et al. 2009; Dubuc et al. 2010; Galvão et al. 2010; Ospina et al. 2010; Martinez-Patino et al. 2011). Dry matter intake has been recognized as an important risk factor for the development of uterine disease. Recent observations show that cows that developed metritis and endometritis had a decrease in dry matter intake up to two weeks before calving (Hammon et al. 2006; Huzzey et al. 2007). This decrease in dry matter intake was accompanied by an increase in NEFA and BHBA in blood, indicating a greater degree of negative energy balance and immunosuppression in those cows (Hammon et al. 2006; Galvão et al. 2010).
Others have tried to find cutoff levels for NEFA and BHBA pre- and postpartum that can determine the risk of cows developing uterine disease postpartum. Duffield et al. (2009) observed that the best cutoff for BHBA at the first week postpartum to predict metritis was >1200 μml/l. Cows with BHBA >1200 μml/l had 2.1x greater likelihood of developing metritis postpartum. Ospina et al. (2010) found a lower cutoff for BHBA in the first two weeks postpartum as a predictor of metritis (>700 μml/l). Dubuc et al. (2010) observed that NEFA concentrations =600 mml/l one week before calving were predictive of metritis postpartum. Ospina et al. (2010) found that NEFA concentrations >360 mml/l either two weeks before or two weeks after calving were predictive of metritis. It is not clear why differences exist in the cutoffs between these two reports because both used Holstein cows and had a similar sample size (about 1,400 cows). The timing of sampling may be the most striking difference. Dubuc et al. (2010) sampled cows one week before calving, which may have resulted in higher concentrations and less variation. In that study (Dubuc et al. 2010), the cutoff for endometritis was found to be =1,100 μml/l of BHBA in the first week postpartum.
Neutrophils mainly depend on glucose uptake and glycolysis for the energy required for chemotaxis, but they almost exclusively depend on glycogen stores for phagocytosis and microbial killing even in the presence of extracellular glucose (Weisdorf, Craddock, and Jacob 1982a; Weisdorf, Craddock, and Jacob 1982b). One recent study found that cows that develop metritis or endometritis had decreased neutrophil glycogen stores around the time of calving, which could be a predisposing factor for uterine disease later in lactation (Galvão et al. 2010).
Hypocalcemia has been consistently associated with RP (Curtis et al. 1983; Curtis et al. 1985; Correa, Erb, and Scarlett 1993), and, in some studies, with metritis (Gröhn et al. 1990). Calcium is a key mediator in several cell processes, including activation of immune cells. In a recent study, cows that developed metritis had decreased calcium concentrations in the first two weeks postpartum, and lower calcium was associated with decreased neutrophil function (Martinez-Patino et al. 2011). Interestingly, the ability to maintain calcium concentration in blood in the first three days after calving was more important than the absolute calcium concentration. It has been found that the greater the drop in calcium concentration in the first three days postpartum, the greater the probability of developing metritis later in lactation (Figure 1).
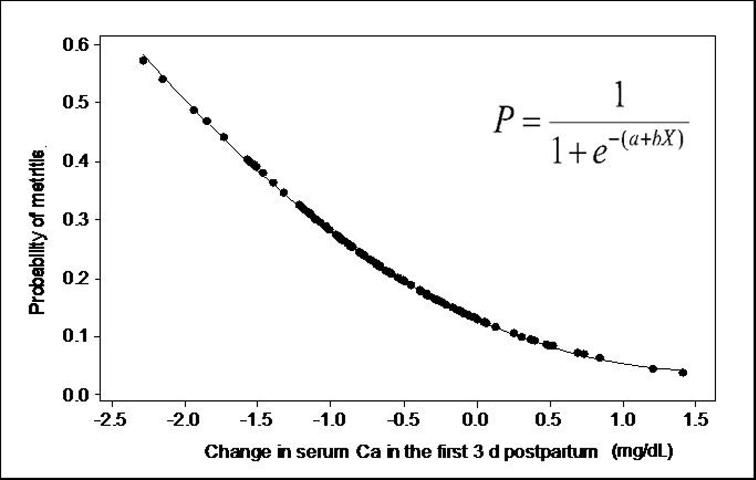
Credit: Martinez-Patino et al. (2011)
Summary
Uterine diseases are highly prevalent in high-producing dairy cows. Risk factors associated with metritis include primiparity, dystocia, twins, RP, stillbirth, abortion, prolapsed uterus, and ketosis. Risk factors for endometritis include dystocia, twins, RP, stillbirth, abortion, metritis, problems with vulval conformation, male offspring, and ketosis. BHBA blood concentration =1,200 μml/l in the first week postpartum is predictive of metritis, while BHBA blood concentration =1,100 μml/l in the first week postpartum is predictive of endometritis. NEFA blood concentration =600 mml/l in the first week postpartum is predictive of metritis. If samples are collected in the first two weeks postpartum, the cutoff for predicting metritis is =700 μml/l for BHBA and =360 mml/l for NEFA. Neutrophil glycogen stores and calcium are associated with the development of uterine disease. Particularly, a drop in calcium in the first three days postpartum is a strong predictor of metritis.
References/Suggested Reading
Cheong, S.H., D.V. Nydam, K.N. Galvão, B.M. Crosier, and R.O. Gilbert. 2011. "Cow-level and Herd-level Risk Factors for Subclinical Endometritis in Lactating Holstein Cows." J. Dairy Sci. 94:762–70.
Correa, M.T., H. Erb, and J. Scarlett. 1993. "Path Analysis for Seven Postpartum Disorders of Holstein Cows." J. Dairy Sci. 76:1305–12.
Curtis, C.R., H.N. Erb, C.J. Sniffen, R.D. Smith, P.A. Powers, M.C. Smith, M.E. White, R.B. Hillman, and E.J. Pearson. 1983. "Association of Parturient Hypocalcemia with Eight Periparturient Disorders in Holstein Cows." J. Am. Vet. Med. Assoc. 183:559–61.
Curtis, C.R., H.N. Erb, C.J. Sniffen, R.D. Smith, and D.S. Kronfeld. 1985. "Path Analysis of Dry Period Nutrition, Postpartum Metabolic and Reproductive Disorders, and Mastitis in Holstein Cows." J. Dairy Sci. 68:2347–60.
Dohoo, I.R., and S.W. Martin. 1984. "Subclinical Ketosis: Prevalence and Associations with Production and Disease." Can. J. Comp. Med. 48:1–5.
Dubuc, J., T.F. Duffield, K.E. Leslie, J.S. Walton, and S.J. LeBlanc. 2010. "Risk Factors for Postpartum Uterine Diseases in Dairy Cows." J. Dairy Sci. 93:5764–71.
Duffield, T.F., K.D. Lissemore, B.W. McBride, and K.E. Leslie. 2009. "Impact of Hyperketonemia in Early Lactation Dairy Cows on Health and Production." J. Dairy Sci. 92:571–80.
Erb, H.N., S.W. Martin, N. Ison, and S. Swaminathan. 1981a. "Interrelationships between Production and Reproductive Diseases in Holstein Cows. Path Analysis." J. Dairy Sci. 64:282–9.
Erb, H.N., S.W. Martin, N. Ison, and S. Swaminathan. 1981b. "Interrelationships between Production and Reproductive Diseases in Holstein Cows. Conditional Relationships between Production and Disease." J. Dairy Sci. 64:272–81.
Galvão, K.N., L.F. Greco, J.M. Vilela, M.F. Sá Filho, and J.E.P. Santos. 2009a. "Effect of Intrauterine Infusion of Ceftiofur on Uterine Health and Fertility in Dairy Cows." J. Dairy Sci. 92:1532–42.
Galvão, K.N., M. Frajblat, S.B. Brittin, W.R. Butler, C.L. Guard, and R.O. Gilbert. 2009b. "Effect of Prostaglandin F2alpha on Subclinical Endometritis and Fertility in Dairy Cows." J. Dairy Sci. 92:4906–13.
Galvão, K.N., M.J. Flaminio, S.B. Brittin, R. Sper, M. Fraga, L. Caixeta, A. Ricci, C.L. Guard, W.R. Butler, and R.O. Gilbert. 2010. "Association between Uterine Disease and Indicators of Neutrophil and Systemic Energy Status in Lactating Holstein Cows." J. Dairy Sci. 93:2926–37.
Gilbert, R.O., Y.T. Gröhn, P.M. Miller, and D.J. Hoffman. 1993. "Effect of Parity on Periparturient Neutrophil Function in Dairy Cows." Vet. Immunol. Immunopathol. 36:75–82.
Gilbert, R.O., S.T. Shin, C.L. Guard, H.N. Erb, and M. Frajblat. 2005. "Prevalence of Endometritis and Its Effects on Reproductive Performance of Dairy Cows." Theriogenology 64:1879–88.
Goshen, T., and N.Y. Shpigel. 2006. "Evaluation of Intrauterine Antibiotic Treatment of Clinical Metritis and Retained Fetal Membranes in Dairy Cows." Theriogenology 66:2210–18.
Gröhn, Y.T., H.N. Erb, C.E. Mcculloch, and H.S. Saloniemi. 1990. "Epidemiology of Reproductive Disorders in Dairy Cattle: Associations among Host Characteristics, Disease and Production." Prev. Vet. Med. 8:25–39.
Hammon, D.S., I.M. Evjen, T.R. Dhiman, J.P. Goff, and J.L. Walters. 2006. "Neutrophil Function and Energy Status in Holstein Cows with Uterine Health Disorders." Vet. Immunol. Immunopathol. 113:21–9.
Huzzey, J.M., D.M. Veira, D.M. Weary, and M.A. von Keyserlingk. 2007. "Prepartum Behavior and Dry Matter Intake Identify Dairy Cows at Risk for Metritis." J. Dairy Sci. 90: 3220–33.
Kaneene, J.B., and R. Miller. 1995. "Risk Factors for Metritis in Michigan Dairy Cattle Using Herd- and Cow-Based Modeling Approaches." Prev. Vet. Med. 23:183–200.
Kasimanickam, R., T.F. Duffield, R.A. Foster, C.J. Gartley, K.E. Leslie, J.S. Walton, and W.H. Johnson. 2004. "Endometrial Cytology and Ultrasonography for the Detection of Subclinical Endometritis in Postpartum Dairy Cows." Theriogenology 62:9–23.
Kasimanickam, R., T.F. Duffield, R.A. Foster, C.J. Gartley, K.E. Leslie, J.S. Walton, and W.H. Johnson. 2005. "The Effect of a Single Administration of Cephapirin or Cloprostenol on the Reproductive Performance of Dairy Cows with Subclinical Endometritis." Theriogenology 63:818–30.
Kehrli, M.E. Jr., B.J. Nonnecke, and J.A. Roth. 1989. "Alterations in Bovine Neutrophil Function during the Periparturient Period." Am. J. Vet. Res. 50:207–14.
Kimura, K., J.P. Goff, and M.E. Kehrli, Jr. 1999. "Effects of the Presence of the Mammary Gland on Expression of Neutrophil Adhesion Molecules and Myeloperoxidase Activity in Periparturient Dairy Cows." J. Dairy Sci. 82:2385–92.
LeBlanc, S.J., T.F. Duffield, K.E. Leslie, K.G. Bateman, G.P. Keefe, J.S. Walton, and W.H. Johnson. 2002. "Defining and Diagnosing Postpartum Clinical Endometritis and Its Impact on Reproductive Performance in Dairy Cows." J. Dairy Sci. 85:2223–2236.
McDougall, S., R. Macaulay, and C. Compton. 2007. "Association between Endometritis Diagnosis Using a Novel Intravaginal Device and Reproductive Performance in Dairy Cattle." Anim. Reprod. Sci. 99:9–23.
Markusfeld, O. 1984. "Factors Responsible for Post Parturient Metritis in Dairy Cattle." Vet. Rec. 114:539–42.
Markusfeld, O. 1985. "Relationship between Overfeeding, Metritis and Ketosis in High Yielding Dairy Cows." Vet. Rec. 116:489–91.
Markusfeld, O. 1987. "Periparturient Traits in Seven High Dairy Herds. Incidence Rates, Association with Parity, and Interrelationships among Traits." J. Dairy Sci. 70:158–66.
Martinez-Patino, N., C.A. Risco, F. Maunsell, K.N. Galvão, and J.E. Santos. 2011. Presentation at the 44th Annual Conference of the American Association of Bovine Practitioners, St. Louis, Missouri, September 22–24, 2011.
Muller, L., and D. Ellinger. 1981. "Colostral Immunoglobulin Concentrations among Breeds of Dairy Cattle." J. Dairy Sci. 64:1727–30.
Nonnecke, B.J., K. Kimura, J.P. Goff, and M.E. Kehrli, Jr. 2003. "Effects of the Mammary Gland on Functional Capacities of Blood Mononuclear Leukocyte Populations from Periparturient Cows." J. Dairy Sci. 86:2359–68.
Ospina, P.A., D.V. Nydam, T. Stokol, and T.R. Overton. 2010. "Evaluation of Nonesterified Fatty Acids and Beta-hydroxybutyrate in Transition Dairy Cattle in the Northeastern United States: Critical Thresholds for Prediction of Clinical Diseases." J. Dairy Sci. 93:546–54.
Potter, T.J., J. Guitian, J. Fishwick, P.J. Gordon, and I.M. Sheldon. 2010. "Risk Factors for Clinical Endometritis in Postpartum Dairy Cattle." Theriogenology 74:127–34.
Weisdorf, D.J., P.R. Craddock, and H.S. Jacob. 1982a. "Glycogenolysis versus Glucose Transport in Human Granulocytes: Differential Activation in Phagocytosis and Chemotaxis." Blood 60:888–93.
Weisdorf, D.J., P.R. Craddock, and H.S. Jacob. 1982b. "Granulocytes Utilize Different Energy Sources for Movement and Phagocytosis." Inflammation 6:245–56.