Knowledge of the soil seed bank composition can contribute to predictive and historical understanding of plant community composition. Possible applications include: 1) determining likely species composition following a disturbance; 2) characterizing local weed population dynamics; 3) predicting the potential contribution of the seed bank to restoration of a target plant community; and 4) determining historical plant community composition.
The seed bank may differ from the current field population. It may contain species not currently found in the field population or may contain the same species in very different numbers than the field population. As some species produce substantially more seeds than others, the seed bank is often dominated by a relatively small number of species. Species that are more likely spread vegetatively may have few or no seeds present in the seed bank. Additionally, species availability in seed banks is affected by the length of time that seeds persist and remain viable. Viability can range from days to decades.
Despite differences between the seed bank and existing vegetation, the seed bank is an indication of the potential species composition at a site. In a restoration context, knowing which species are available in the seed bank can help natural resource managers make decisions about what species may need to be planted after a disturbance in order to have the desired species represented.
The purpose of this publication is to summarize standard techniques for wetland seed bank assays, including greenhouse set-up, sample collection and sample processing. Environmental conditions and "ponded" bench construction are presented first since these must be addressed prior to sampling (sample storage time may need to be minimized). The methodologies below are applicable in any climate, but care has been taken to mention specific considerations and references for warmer climates (particularly Florida).
Environmental Conditions for Seed Bank Assays
Temperature and photoperiod are two environmental factors important to plant germination and growth (Baskin and Baskin 2001). When conducting a seed bank assay, the greenhouse (or other experimental site) should be maintained at light cycles and temperature ranges similar to the field site until the assay is completed (Harwell and Havens 2003; Smith et al. 2002; Williges and Harris 1995; Looney and Gibson 1995). Mimicking field conditions in the greenhouse will facilitate seed bank emergence that is typical of field conditions, and will also limit confounding variables in the assay.
If the temperature in the assay location is different from that in the field location, the difference should be minimized with heating or cooling (see Mahaney et al. 2004). Maintaining cooler temperatures can be a challenge for greenhouses during warmer months. If climate control is not available, cooling approaches can include a combination of an evaporative cooling system, and overhead and wall ventilation fans. During colder periods, maintain inside air temperature by covering evaporative cooling pads with an interior plastic sealing flap. This flap prevents most of the cooler outside air from entering the greenhouse, but allows for air exchange. Heating approaches can include an overhead heater tube connected to a furnace, and circulation fans.
If the photoperiod or light intensity differs between the greenhouse and the study site, augment natural light with artificial lighting (use a light meter to compare light intensity in the field and greenhouse). For example, seeds transported from Florida to Iowa received additional light from high-intensity bulbs matched to the photoperiod of the Florida field site (van der Valk and Rosburg 1997). Harwell and Havens (2003) used fluorescent tubes with wattages reflective of light data taken in the field to provide natural photoperiod and light levels to their submerged, aquatic vegetation seed bank assay. Natural photoperiod was maintained and natural light conditions achieved with the use of supplementary lighting in a number of seed bank studies (Mahaney et al. 2004; ter Heerdt et al. 1996; Boedeltje et al. 2002; and Combroux et al. 2001 and 2002).
In order to monitor seed bank assay conditions in the greenhouse, temperature and relative humidity data can be taken regularly (i.e., every 15 minutes) using a HOBO Pro Series temperature/relative humidity data logger. The HOBO should be enclosed in a solar radiation shield to protect it from solar radiation and reflected heat, and improve the measurement accuracy.
Ponded Benches Provide Optimal Wetland Emergence Conditions (for Saturated Soils)
One method of creating wetland conditions in a greenhouse is to construct a "ponded" bench for the emergence assay. To create your own ponded bench from an existing greenhouse bench:
-
Level an existing bench. Small differences in bench elevation can result in large differences in the hydrology of the trays in the benches. These differences will confound the assay.
-
After the bench is leveled, cover the entire top surface of the bench with plywood (Figure 1A).
-
Create a lip around the perimeter of each bench by attaching 2-inch by 4-inch boards to the surface (Figure 1B).
-
For bench drainage and cleaning, drill three holes through the plywood along the long side of the bench. The holes should be the same size as the drains being installed, and should be positioned near the edge of the bench. Drains and stoppers can be purchased at any hardware store.
-
Line the bench with a single sheet of ethylene propylene diene monomer rubber (EPDM) that is larger than the surface area of the bench by a few feet in all directions (Figure 1C and D). EPDM can be purchased in various sizes and shapes from internet sites specializing in pond liners and water gardens.
-
Mark the underside of the EPDM at the places where the drains are located. Make a vertical and horizontal slit in the EPDM that is smaller than the drain to be installed. Lay the EPDM back in place. With the EPDM acting as a washer, the drain can be screwed together and plugged with a stopper to create a water-tight seal.
-
Fill the bench with water. A "Little Giant" automatic stock tank siphon float valve can be modified and installed on the bench to automatically maintain water at the desired level (Figure 1E). The "Little Giant" supplies water to each bench through a garden hose coupled to a back-flow preventer.
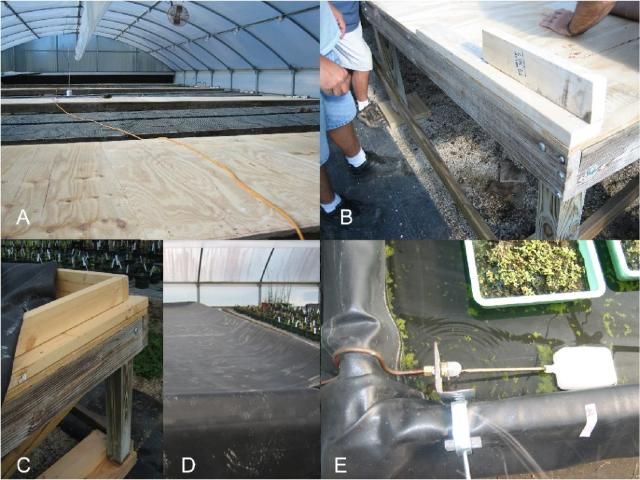
Credit: Nancy Steigerwalt
Seed Bank Sample Collection
A stratified random sampling design is commonly used for seed bank sampling when differences in the seed bank are anticipated (e.g., vegetation zones, hydrologic zones, soil zones, land-use history, etc.) (van der Valk 1978; Williges and Harris 1995; Poiani and Johnson 1988). For stratified random sampling, divide the study area into smaller units (subpopulations) representing anticipated differences, and collect samples randomly within each smaller unit (McCune and Grace 2002).
To account for the high spatial variability characteristic of many seed banks, it is preferable to take many small samples rather than a few large samples (Roberts 1981). Also, a number of small samples can be collected and composited (Bigwood and Inouye 1988); for example, collecting five cores within a 5-square-meter area.
A tulip bulb planter is frequently used to collect seed bank samples; however, any sampler that allows for sampling a known volume will work. Seed bank samples are typically collected to a depth of 5 centimeters (Galatowitsch and van der Valk 1996; Poiani and Johnson 1988; Harwell and Havens 2003) because this region of the soil typically contains the highest density of viable seeds (Roberts 1981). However, it is not uncommon to take deeper soil cores to answer research questions.
Seed Bank Sample Processing
Seed bank samples should be kept separate in airtight bags and stored cool, but not frozen (i.e., on ice in a cooler) during transport. In order to keep seeds dormant until the emergence assay is set up, store samples in a dark refrigerator or cold room (Miao et al. 2001; Leeds et al. 2002; Williges and Harris 1995; Hanselman et al. 2005; Looney and Gibson 1995). To ensure that all emerging plants are seed-source, remove any material that could vegetatively propagate (rhizomes, roots, and any remaining live plant tissue) and manipulate the soil through a screen (6.35 mm wire mesh is one choice) (Figure 2A).
Shallow containers (e.g., Polystyrene Perma-Nest plant trays, 20 x 20. x 6 centimeters) make excellent containers for seed bank assays and are available from a variety of merchants. If the containers do not have pre-drilled holes, drill 5 holes in the bottom of each tray to allow for water circulation. Fill each tray with a 2-centimeter porous layer using coconut coir fiber potting medium, sand, or absorbent rockwool (Figure 2B). Filling the bottom of the tray with porous material prevents gas formation and algal blooms that would otherwise occur in submerged trays (Boedeltje et al. 2002; Barko and Smart 1983). Fill each tray with 2 centimeters of potting soil over the porous material. Spread the sifted seed bank sample on top of the potting soil evenly to a depth of 1 centimeter (Figure 2C).

Credit: A) by Carrie Reinhardt Adams; B, C and D) by Nancy Steigerwalt
Setup for Seed Bank Assay
Randomly distribute the trays in your greenhouse bench to avoid any bias in placement (Figure 2D). Not randomizing the trays can add a confounding variable to the assay. A common approach for seed bank assays is to set up two replicate assays, one with samples maintained at saturated water depths for emergent plants, and one with samples maintained at flooded water depths for submerged plants (Miao et al. 2001; Williges and Harris 1995; van der Valk and Rosburg 1997). Boedeltje et al. (2002) found that the 0- to 1-centimeter water depth ratio germinated both submerged and emergent plants, making the duplication unnecessary, and considerably reducing the space and effort needed to complete the assay. If the research does not include emergent plants, Boedeltje's (2002) method is most efficient. To follow Boedeltje's method, fill the bench with water to a depth within 0 to 1 centimeter of the tray's soil surface. Water level should be maintained within this ratio as it provides adequate water for seed emergence of both submerged and emergent plants (Boedeltje et al. 2002). Failure to maintain consistent water levels for all trays will confound the assay and has the potential to alter the outcome of the assay.
Seed from sources other than the sample (most likely greenhouse weeds) may contaminate the assay. To control for seed input, fill a number of trays with only potting medium (no seed bank sample) and place these trays randomly in the bench along with the seed bank trays. If plants emerge from the empty trays, you can determine the potentially contaminating species, and estimate the frequency of contamination.
Seed Bank Emergence and Identification
Remove emerged plants from seed bank trays when plants appear sufficiently established to be able to survive transplant (Figure 3). Transplant each emerged plant into a single labeled pot. Label each pot with the seed bank subsample from which the plant was removed, a number (starting from 1 and going up consecutively) and either M or D to indicate monocot or dicot. Grow unknown plants in a ponded bench until they are mature enough for positive identification. Flowers are usually necessary for identification, and flowering may be induced using fertilization or other means. Reserving all specimens that have tentatively been identified as similar allows for confirmation of identification as plants mature and become more easily identified.

Credit: Nancy Steigerwalt
Length of the Seed Bank Assay
In temporate regions, the majority of seeds germinate and emerge in less than 5 months (Mahaney et al. 2004; Combroux et al. 2001; Haag 1983). Due to the longer growing period in Florida, it is appropriate to conduct seed bank assays for 6 to 9 months (Leeds et al. 2002; Hanselman et al. 2005; Looney and Gibson 1995). Although emergence can be completed during this time period, the total length of the assay will likely be extended to include the time needed to grow unknown species to maturity for identification.
After the seed bank assay continues beyond the first few weeks, it is likely that some mold, algae, and/or moss may develop on the surface of the seed bank soil sample. This growth might act as a physical barrier to emergence. To minimize this problem, maintain the water level below the soil surface. Should significant mold, algae, and/or moss accumulate, periodically slice through the material with a razor blade and otherwise disturb the soil surface to provide space for seedlings to emerge (Anne Frances, personal communication).
Conclusion
A seed bank assay can provide valuable information regarding the viable seed population in the soil. Careful consideration of potentially confounding factors can prevent bias in seed bank assay results. Although seed bank assay methodology was initially developed in temperate environments, simple adaptations to common methodology allow them to be conducted in varying climates.
Literature Cited
Barko, J. W. and R. M. Smart. 1983. "Effects of organic matter additions to sediment on the growth of aquatic plants." Journal of Ecology 71: 161–175.
Baskin, C. C. and J. M. Baskin. 2001. Seeds: Ecology, Biogeography, and Evolution of dormancy and germination. San Diego, CA: Academic Press.
Bigwood, D. W. and D. W. Inouye. 1988. "Spatial pattern analysis of seed banks: An improved method and optimized sampling." Ecology 69: 497–507.
Boedeltje, G., G. N. J. ter Heerdt, J.P. Bakker. 2002. "Applying the seedling-emergence method under waterlogged conditions to detect the seed bank of aquatic plants in submerged sediments." Aquatic Botany 72: 121–128.
Combroux, I., G. Bornette, N. J. Willby, and C. Amoros. 2001. "Regenerative strategies of aquatic plants in disturbed habitats: The role of the propagule bank ." Archiv fuer Hydrobiologie 152: 215–235.
Combroux, I., G. Bornette, and C. Amoros. 2002. "Plant regenerative strategies after a major disturbance: The case of a riverine wetland restoration." Wetlands 22: 234–246. Anne Frances. personal communication.
Galatowitsch, S. M. and A. G. van der Valk. 1996. "The vegetation of restored and natural prairie wetlands." Ecological Applications 6: 102–112.
Haag, R. W. 1983. "Emergence of seedlings of aquatic macrophytes from lake sediments." Canadian Journal of Botany 61: 148–156.
Hanselman, J. A., M. B. Bush, and M. A. Lee. 2005. "Seed content and percent organic matter in surface sediments as indicators of wetland plant communities, Blue Cypress Marsh, Florida." Florida Scientist 68: 250–260.
Harwell, M. C. and K. E. Havens. 2003. "Experimental studies on the recovery potential of submerged aquatic vegetation after flooding and desiccation in a large subtropical lake." Aquatic Botany 77: 135–151.
Leeds, J. A., S. M. Smith, and P. B. Garrett. 2002. "Seedbanks and their potential role in the vegetation dynamics of a northern Everglades Marsh." Florida Scientist 65: 16–34.
Looney, P. B. and D. J. Gibson. 1995. "The relationship between the soil seed bank and above-ground vegetation of a coastal barrier island." Journal of Vegetation Science 6: 825–836.
Mahaney, W. M., D. H. Wardrop, and R. P. Brooks. 2004. "Impacts of sedimentation and nitrogen enrichment on wetland plant community development." Plant Ecology 175: 227–243.
McCune, B. and J. B. Grace. 2002. Analysis of Ecological Communities. Gleneden Beach, OR: MjM Software Design.
Miao, S. L., P. V. McCormick, S. Newman, and S. Rajagopalan. 2001. "Interactive effects of seed availability, water depth, and phosphorus enrichment on cattail colonization in an Everglades wetland." Wetlands Ecology and Management 9: 39–47.
Poiani, K. A. and W. C. Johnson. 1988. "Evaluation of the emergence method in estimating seed bank composition of prairie wetlands." Aquatic Botany 32: 91–97.
Roberts, H. A. 1981. Seed banks in soil. Advances in Applied Biology 6: 1–55.
Smith, S. M., P. V. McCormick, J. A. Leeds, and P. B Garrett. 2002. "Constraints of seed bank species composition and water depth for restoring vegetation in the Florida Everglades, U.S.A." Restoration Ecology 10: 138–145.
ter Heerdt, G. N. J., G. L. Verweij, R. M. Bekker, and J. P. Bakker. 1996. "An improved method for seed-bank analysis: seedling emergence after removing the soil by sieving." Functional Ecology 10: 144–151.
van der Valk, A. G. 1978. "The role of seed banks in the vegetation dynamics of prairie glacial marshes." Ecology 59: 322–335.
van der Valk, A. G. and T. R. Rosburg.1997. "Seed bank composition along a phosphorus gradient in the northern Florida Everglades." Wetlands 17: 228–236.
Williges, K. A. and T. T. Harris. 1995. "Seed bank dynamics in the Lake Okeechobee marsh ecosystem." Ergebnisse der Limnologie 45: 79–94.