The Florida SLE Mosquito, Culex (Culex) nigripalpus Theobald (Insecta: Diptera: Culicidae)
The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Culex nigripalpus mosquitoesare the most important disease vectors in Florida. They are the proven primary enzootic (normal level of virus transmission from mosquitoes to wild birds) and epidemic (unusually high level of virus transmission from mosquitoes to humans) vectors of St. Louis encephalitis SLE virus throughout the southern half of the state. In addition, they are likely involved in the transmission of eastern equine encephalitis (EEE) virus in Florida, from Titusville north to Jacksonville and west to Pensacola.
Distribution
Culex nigripalpus mosquitoes have a subtropical distribution. They are found in the southern United States, the Caribbean, Mexico, Central America, and northern South America. In the US, this species is found in warm, humid coastal habitats from North Carolina to Texas and up the Mississippi River basin as far north as Kentucky.
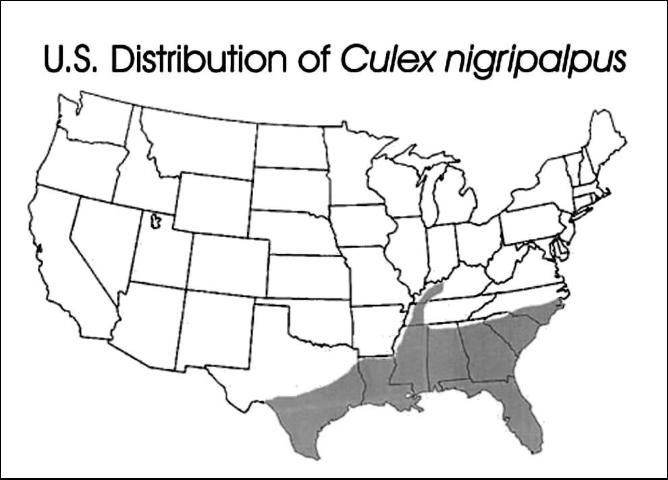
Credit: Carpenter and LaCasse, 1955 edition of Mosquitoes of North America
This species is most abundant in southern coastal regions where winter temperatures are mild. During warm periods, the species will expand its range northward, but adults and larvae are killed when they are exposed to extended periods of below-freezing temperatures. In Florida, this species is found in all 67 counties. However, it is most abundant in the central portion of the peninsula from Brevard County south to Dade County, west across the state to Collier County and north along the Gulf of Mexico to Pensacola.
Description
Culex nigripalpus is a medium-sized mosquito. The proboscis is dark and the palpi short. The scutum is brown with fine dark bronze to brown scales. The pleura has few or no scales. Abdominal tergites are covered with dark-brown to black scales with bronze to metallic blue-green reflection. Narrow white basal bands are occasionally present on the dorsal surface of some abdominal segments. Legs are dark with reflective bronze to metallic blue-green scales. The posterior surface of the femora and tibiae are pale. In general, this is a dark brown, nondescript species with no obvious distinguishing characteristics, making field identification to the species level extremely difficult.
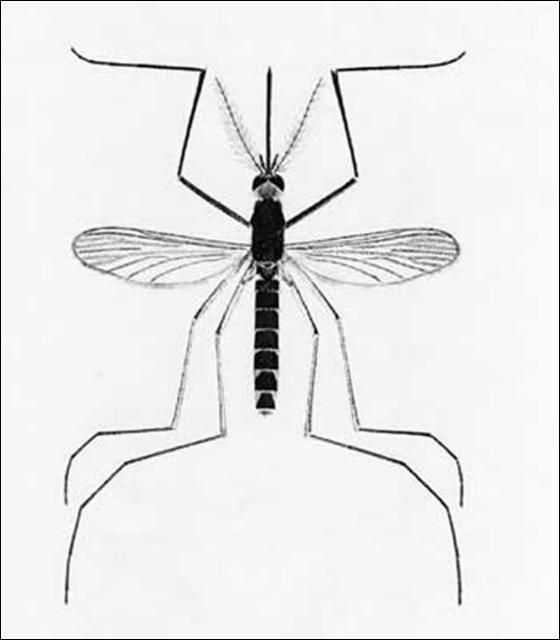
Credit: Carpenter and LaCasse, 1955 edition of Mosquitoes of North America
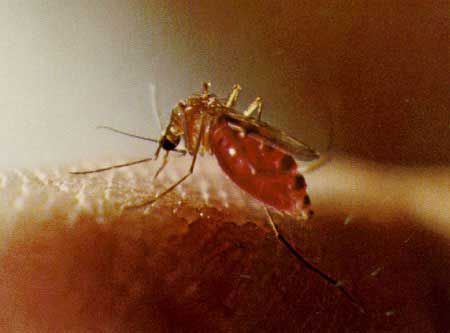
Credit: Jai Nayar, University of Florida
Life Cycle
Gravid Culex nigripalpus females take flight during evening crepuscular periods and search throughout the night for oviposition sites. Eggs of this species have been reported in virtually every type of aquatic habitat. However, the species is best described as a flood water Culex. Females prefer to lay eggs in freshly flooded roadside ditches and agricultural furrows, habitats that flood infrequently and, once flooded, remain wet for 10 to 14 days before drying down completely. Eggs are cemented together loosely and laid in rafts containing from 90 to 210 eggs. They hatch 24 to 36 hours after being laid.
Larvae develop in the rich organic mixture found in shallow flooded ditches. Larval development time is temperature dependent, and is most rapid in mid-summer when the water temperature in the ditches may exceed 100 degrees F. Larvae develop through four instars, each lasting approximately 24 hours in summer and 48 hours in winter. Fourth instar larvae molt to form pupae from which the adult mosquito emerges 36 to 48 hours later.
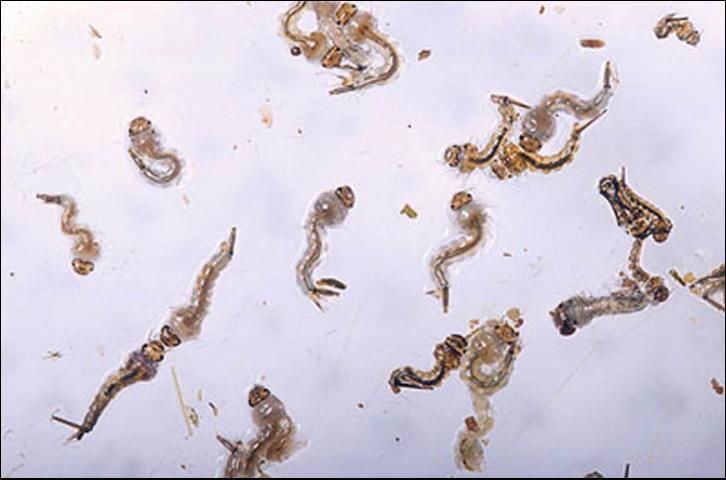
Credit: Peggy Greb, USDA
Adults mate near the breeding site and females begin host seeking when they are approximately 48 hours old. Females are extremely opportunistic in their choice of a host, feeding on vertebrates ranging from tree frogs to humans. Females seek hosts and blood feed mainly during the crepuscular periods before sunrise and after sunset. Host selection is influenced by the relative abundance of hosts found in the mosquito's foraging habitat. During dry winter and spring months, females host seek primarily in lush, humid forest floors and canopies where they usually encounter and feed on resting birds. During wet periods in the summer and autumn, females can forage in open fields and in other habitats from which they were previously excluded because of the dry conditions. In these open habitats, female mosquitoes locate and blood feed on mammalian species such as horses, cows, dogs, rabbits, armadillos, and humans.
Once blood is obtained, it takes the mosquito about four days to digest the meal (again, this is temperature dependent with faster digestion occurring during the hot summer months) and form a batch of eggs. Once eggs are formed, they are laid as soon as a suitable oviposition site is located.
Medical Significance
Culex nigripalpus is the most important disease vector in Florida where it is the predominant vector of SLE virus and a minor vector of EEE virus. During all of the major Florida SLE epidemics, 1959, 1961, and 1962 in the Tampa Bay area, and 1977 and 1990 in the central region of the state, Culex nigripalpus proved to be the only mosquito vector of consequence relative to human SLE transmission. Most of the EEE virus isolates from mosquitoes have been from Culiseta melanura (Coquillett), the known enzootic vector of the EEE virus to wild birds. In addition, a large number of EEE virus isolates in Florida have come from Cx. nigripalpus females.
One reason this species is an efficient arboviral vector is due to its "egg dumping" behavior. Because females prefer to lay their eggs in freshly flooded ditches, there are often long periods, especially during summer droughts, when there are few available oviposition sites. During these periods, the number of gravid females builds steadily, sometimes accounting for up to 90% of the adult female population. After a rainfall sufficient to flood roadside and agricultural ditches (usually 2 or more inches in 24 hours), gravid females oviposit quickly, seek hosts and blood feed in a cycle synchronized by the rainfall event. If the period of drought preceding the rainfall was sufficiently long to allow infected mosquitoes time to complete the extrinsic incubation of the SLE virus (10 to 14 days), then many of the mosquitoes will be infective and able to transmit SLE virus during the blood meal following oviposition. The shear abundance of this species in south Florida (it is found from urban parks to rural farms throughout the year), along with its egg dumping and mass blood feeding behaviors, make it an exceptionally dangerous mosquito species relative to arboviral transmission.
Surveillance and Managment of Culex nigripalpus
Due to the medical significance of this species, vector surveillance, with the ultimate goal of predicting human cases of SLE virus, is of critical importance. Historically, New Jersey light traps and CDC miniature light traps have been used to monitor the abundance of Cx. nigripalpus adults. However, we have found that resting collections made with ground aspirators provide a superior measure of Cx. nigripalpus adult populations. This is because males as well as females in all gonotrophic conditions (host-seeking, blood-fed, partially- blood-fed and gravid) are taken in aspirator collections. Due to their green color, newly emerged adults of both sexes can be identified and counted, thus providing an accurate measure of adult emergence.
Because Cx. nigripalpus females prefer to lay their eggs in freshly flooded ditches, huge numbers of adults are often observed during the wet summer months in south Florida. The vast number of breeding habitats in which this species is found results in adult populations that are difficult to regulate and thus this species is seldom targeted for control efforts. A notable exception occurs during periods of known or suspected SLE transmission. These transmission cycles are usually identified by increases in sentinel chicken or wild bird antibody seroconversions to SLE virus or by the occurrence of confirmed human SLE cases. During SLE epidemics, control efforts directed against Cx. nigripalpus females are sometimes undertaken. Because of the overwhelming number of oviposition sites, larval control is seldom attempted.
Ultra Low Volume (ULV) adulticide applications of malathion, naled or fenthion by ground vehicles can be directed against Cx. nigripalpus adults, especially in areas such as hospitals and retirement villages, where humans that are more susceptible to SLE infection reside. Occasionally, ULV applications of adulticides are made from the air. Because of the expense, when county-wide broadcast applications of aerial ULV treatments are attempted, an effort is sometimes made to coordinate aerial insecticide applications with the rainfall-driven oviposition cycles of gravid females. This technique maximizes the kill of older gravid and blood-seeking females that are most capable of transmitting the SLE virus. To date, there is little evidence that ULV adulticide applications, made from the ground or from the air, control Cx.nigripalpus females or interrupt SLE transmission cycles in Florida.
Selected References
Dame D, Fasulo TR. (2003). Mosquitoes. Public Health Pesticide Applicator Training Manual. http://entomology.ifas.ufl.edu/fasulo/vector/ (21 January 2004)
Day JF. 1991. Epidemic proportions. Natural History. July, 50–53.
Day JF, Curtis GA. 1993. Annual emergence patterns of Culex nigripalpus females before, during and after a widespread St. Louis encephalitis epidemic in south Florida. Journal of American Mosquito Control Association 9: 249–255.
Day JF, Curtis GA. 1994. When it rains, they soar—and that makes Culex nigripalpus a dangerous mosquito. American Entomologist 40: 162–167.
FMEL. (2019) Identification guide to common mosquitos of Florida. https://fmel.ifas.ufl.edu/mosquito-guide/. (24 March 2021).



