Introduction
Hygrophila polysperma (Roxb.) T. Anderson (Polemoniales: Acanthaceae) is a rooted submersed or emersed aquatic plant in shallow water areas and saturated shorelines throughout Florida. This invasive aquatic plant also is known as hygrophila, hygro, East Indian hygro, green hygro, Miramar weed, oriental ludwigia, and Indian swampweed (hereafter referred to as hygrophila).
Hygrophila is a federally listed noxious weed (USDA 2012), a Florida state listed Category II prohibited plant (FDACS 2008), and a Florida Exotic Pest Plant Council Category I invasive species (FLEPPC 2019). The submersed growth habit displaces native vegetation in many canals and drainage ditches in south Florida. The plant forms dense strands that occupy the entire water column, clogging irrigation and flood-control systems (Schmitz and Nall 1984, Sutton 1995) and interfering with navigation (Woolfe 1995). Hygrophila also creates problems as an emergent plant in some shoreline areas, including rice fields (Krombholz 1996).
In October 2007, we received a report from researchers at the UF/IFAS Center for Aquatic and Invasive Plants of an insect attacking hygrophila. Samples of the insect were collected, and it was identified as the waterlily leafcutter, Elophila obliteralis (Walker). Of the more than twenty Acentropinae species occurring in Florida, Elophila obliteralis (Walker) is the most common. Although its common name implies that it is a pest of waterlilies, it actually has a wide host range. Most of the damage caused by the larvae is superficial and rarely endangers the plant, but the damage observed on the hygrophila plants in this instance was severe (Figures 1 and 2).

Credit: J. P. Cuda, University of Florida

Credit: J. P. Cuda, University of Florida
In addition to the invasive aquatic weed hygrophila, the waterlily leafcutter also feeds on another invasive plant, hydrilla, Hydrilla verticillata (L.f.) Royle. Numbers of Elophila obliteralis collected from hydrilla from field sites in Florida and Louisiana were similar to the numbers of the hydrilla leafcutter, Parapoynx diminutalis Snellen that were collected (Balciunas and Minno 1985).
Synonomy
According to Dyar (1906) and Zimmerman (1958) the following synonyms have been used for Elophila obliteralis:
Synclita obliteralis Walker, 1859
Synclita proprialis Fernald, 1859
Isopteryx obliteralis Walker, 1859
Parapoynx obscuralis Walker, 1859
Parapoynx obscuralis M?schler, 1972
Hydrocampa proprialis Fernald, 1888
Hydrocampa obliteralis Fernald, 1891
Nymphula obliteralis Hampson, 1897
Distribution
This common moth occurs throughout Florida, westward to Texas, northward to western Nova Scotia, and southern Manitoba (Munroe 1972). It also has been introduced into Hawaii (Williams 1944), England (Shaffer 1968), and British Columbia (Munroe 1972). In a study in South Carolina, Elophila obliteralis was found in both lentic (still freshwater) and lotic (fast moving freshwater) water bodies (Stoops et al. 1998). Out of 65 surveyed sites in South Carolina, Elophila obliteralis was present in 6.2% of those sites with a water temperature between 23 and 27°C, a width of 3.2 to 45.7 m, a depth of 0.3-1.0 m, and a pH of 6.6 to 7.5 (Stoops et al. 1998).
Description
Eggs
The eggs are whitish in color and appear domelike (oval and flattened). The flattened side is glued to the leaf and the domed side has wrinkles down the length of the egg (Dyar 1906). The eggs are 0.6 mm in length and 0.4 mm wide (Dyar 1906). They are deposited singly or in overlapping, ribbon-like masses near the edges of submersed leaf surfaces.
Larvae
Most members of the crambid subfamily Acentropinae have aquatic larvae with tracheal gills. However, the larva of this moth lacks gills and is sometimes referred to as "the sandwich man" due to its habit of living between two pieces of leaf (leaf case) that it cuts from its host plant (Figure 3 and 4).

Credit: Lyle J. Buss, University of Florida
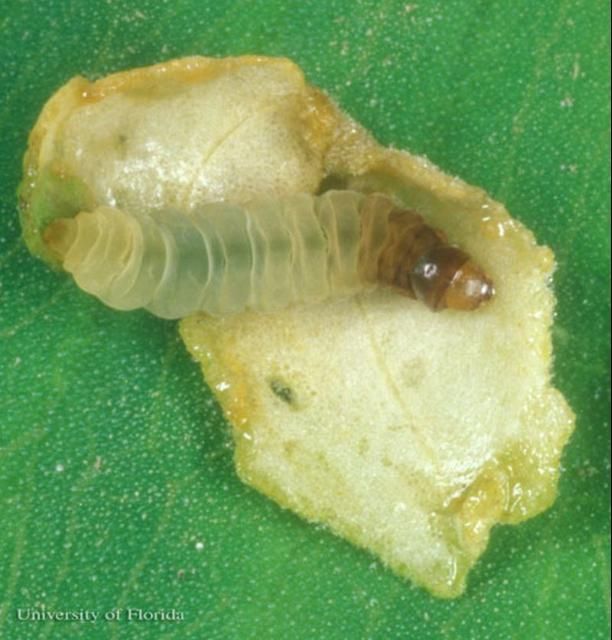
Credit: Lyle J. Buss, University of Florida
The epidermis (skin) of the larvae is covered with minute papillae (bumps). The body is creamy-white, but increasingly brownish from abdominal segment four forward to the prothorax. The prothoracic coxae (proximal leg segments) are touching while the mesothoracic coxae are nearly touching. The head is yellowish-brown with a faint brown genal (cheek) stripe. The prothoracic spiracle (respiratory opening) is vestigial (non-functioning), and the spiracles on abdominal segments three and four are distinctly larger than others. The crochets (gripping hooks) are arranged in two biordinal (sometimes partially triordinal) transverse bands, with the anterior band distinctly larger than the posterior band.
Pupae
The pupae are pale yellow and the wings and head appear darker (Figure 5) (Dyar 1906). The head has two distinct black spine-like hairs. The spiracles on abdominal segments 2–4 are large, round, elevated and red brown in color (Dyar 1906). The anterior spiracles are much smaller. The pupae are found inside silk cocoons inside the leaf cases formed by the larvae.

Credit: Stephen P. L. Luk
Adults
Adults are sexually dimorphic and readily distinguishable (Figures 6 and 7). Females have a 15 to 19 mm wingspan, and the female's wings are paler in color appearing grayish-brown with orange-brown markings. The wingspan of the male is smaller, about 11 to 13 mm, and the male's wings are grayish-brown interspersed with brownish and white markings.

Credit: J. Lotz, Division of Plant Industry

Credit: L. J. Buss, University of Florida
Life Cycle and Biology
No published information is available about the development times of this species. The female moth lays her eggs on the exposed edges of submersed aquatic plants (Gill et al. 2008). Upon hatching, the larvae enclose themselves inside cut leaf pieces. The leaves are webbed together with silk. Cases made by young larvae are water-filled and oxygen uptake occurs cutaneously (presumably via the epidermal papillae), whereas cases of older larvae are air-filled. The cases of young larvae remain attached to the leaf from which they were made. Older larvae detach the case from the leaf and are free-floating. Larvae abandon smaller cases as they mature and construct larger cases from new leaves. The case may consist of two entire leaves, parts of leaves, or of parts of many plants tied together with silk. The larvae extend out of the case to feed on plant material, but usually the body remains in the case. Prior to pupation, larvae attach their cases to petioles or leaf blades of their host plants above or below the water surface and spin a silk cocoon inside their leaf cases.
Hosts
Elophila obliteralis has a wide host range and is known to feed on nearly 60 plant species (Table 1).
Host plants of the waterlily leafcutter, Elophila obliteralis (Walker). Plant hosts arranged by families and genera.
Damage
Elophila obliteralis has a wide host range and is known to feed on waterlilies and other ornamental pond plants as well as the invasive aquatic weeds, salvinia (Tipping et al. 2012), water lettuce (Dray et al. 1993), hygrophila, and hydrilla. The larvae are the stage that feeds on the plant and causes damage to the plant tissue. In addition to feeding, the larvae cut the leaves to prepare a leaf case for shelter. As the larvae develop, they cut new, progressively larger leaf cases. This action in itself can provide quite significant damage to the infested plant (Nachtrieb et al. 2007). In a field study, to compare the effect of herbivory on different aquatic plants, Elophila obliteralis was one of the three species that caused the most damage (Nachtrieb et al. 2007). When feeding the larvae remove chunks from the leaves, usually feeding on the basal or middle portions (Balciunas and Minno 1985). This feeding often causes the leaves to break away from the stem. If the population density is high and plant material becomes scarcer, the larvae will begin to feed on the stems, which can cause the entire plant to fragment (Balciunas and Minno 1985).
Due to its broad host range, this insect frequently is a pest in aquatic plant nurseries, especially on waterlilies, Nymphaea spp. In the nursery setting, this insect can cause economic losses as the larval feeding makes the plants unattractive to customers. Extensive feeding may even lead to reduced plant health and death (Gill et al. 2008).
Importance as a Biological Control Agent
In addition to having a pest status in aquatic nurseries, due to its wide host range, Elophila obliteralis also plays a minor role as a beneficial insect, as it feeds on invasive species, such as hydrilla, salvinia, and hygrophila. As a native species this type of biological control is known as natural regulation. However, due to the extensive host range of this species it would not be advisable to attempt to increase wild numbers through mass releases or conservation, as they would likely feed non-specifically on other desirable plants as well as the weeds.
A study that attempted to identify the biotic and abiotic factors that limited growth of common salvinia, an exotic floating aquatic plant, found that herbivory by Elophila obliteralis and a weevil, Cyrtobagous salviniae Calder and Sands, were two of the most influential factors inhibiting growth (Tipping et al. 2012).
Monitoring and Management
To monitor for the waterlily leafcutter, observe leaves for the characteristic holes created by this insect (Gill et al. 2008). The adults can be trapped by UV black lights, and the larvae can be extracted from the plant material by handpicking or using a Berlese funnel.
Elophila obliteralis is a pest of greenhouses and may require control in aquatic plant nurseries. As with other aquatic moth pests, Bacillus thuringiensis subspecies kurkstaki would likely provide control with little or no adverse effects to other aquatic organisms (see Baniszewski et al. 2016). In support of this hypothesis, the closely related organism Bacillus thuringiensis subspecies israelensis was found to cause significant mortality to the waterlily leafcutter (Haag and Buckingham 1991).
Related Species
Three other species of Elophila occur in the United States with one, Elophila tinealis Munroe, in Florida. The adult of Elophila tinealis is much smaller than that of the waterlily leafcutter and has longer, narrower, and darker wings. The larvae of Elophila tinealis are not well known, but seem to feed on and most often make their cases out of duckweed, Lemna sp.
The larvae of Elophila gyralis (Hulst) and Elophila icciusalis (Walker) are similar to those of the waterlily leafcutter, but the anterior and posterior transverse bands of crochets (i.e., the gripping hooks on the prolegs) are the same size. Elophila gyralis and Elophila icciusalis adults are more brightly colored than Elophila and are yellowish-orange and white or brownish in color. Although Elophila gyralis and Elophila icciusalis larvae may make portable cases, they usually cut only one leaf piece and attach it to a whole leaf and live between the two layers.
Selected References
Balciunas JK, Minno MC. 1985. Insects damaging hydrilla in the U.S.A. Journal of Aquatic Plant Management 23: 77–83.
Baniszewski J, Weeks ENI, Cuda JP. 2016. Bacillus thuringiensis subspecies kurstaki reduces competition by Parapoynx diminutalis (Lepidoptera: Crambidae) in colonies of the hydrilla biological control agent Cricotopus lebetis (Diptera: Chironomidae). Florida Entomologist 99: 644–647.
Dray FA, Center TD, Habeck DH. 1993. Phytophagous insects associated with Pistia statiotes in Florida. Environmental Entomology 22: 1146–1155.
Dyar HG. 1906. The North American Nymphulinae and Scopariinae. Journal of the New York Entomological Society 14: 77–108.
[FDACS] Florida Department of Agriculture and Consumer Services. (2008). Aquatic Plant Importation Transportation, Non-nursery Cultivation, Possession, and Collection: Prohibited Aquatic Plants. [E1] FDACS, Division of Plant Industry, Gainesville, FL, USA (23 April 2020).
[FLEPPC] Florida Exotic Pest Plant Council. (2019). List of Florida's Invasive Species. [E2] Florida Exotic Pest Plant Council Invasive Plant Lists (23 June 2020).
Gill S, Reeser, R, Raupp, M. 2008. Controlling two aquatic plant pests Nymphuliella daeckealis (Haimbach) and the waterlily leafcutter, Synclita obliteralis (Walker). The University of Maryland Cooperative Extension factsheet 818. 7 pages.
Haag KH and Buckingham GR. 1991. Effects of herbicides and microbial insecticides on the insects of aquatic plants. Journal of Aquatic Plant Management 29: 55–57.
Krombholz P. 1996. Hygrophila polysperma: an indicator plant. The Aquatic Gardener: Journal of the Aquatic Gardeners Association 9: 135–137.
Munroe E. 1972. Fasicle 13.1A: Pyraloidea: Pyralidae (part). The Moths of America North of Mexico (MONA) Series (Dominick RB et al. [eds]). EW Classey Ltd and R.B.D. Publications Inc., London, UK. 134 pages.
Nachtrieb JG, Grodowitz MJ, Smart RM. 2007. Impact of invertebrate herbivory on native aquatic macrophytes. Aquatic Plant Control Research Program Technical Notes Collection. Vicksburg, MS: U.S. Army Engineer Research and Development Center. ERDC/TN APCRP-BC-9..
Schmitz DC, Nall LE. 1984. Status of Hygrophila polysperma in Florida. Aquatics 6: 11–14.
Schmitz DC, Nelson BV, Nall JE, Schardt JD.1991. Exotic aquatic plants in Florida: a historical perspective and review of the present aquatic plant regulation program. pp. 303–326. In Center TC, Doren RF, Hofstetter RL, Myers RL, Whiteaker LD (eds.), Proceedings of a symposium on exotic pest plants. Technical Report NPS/NREVER/NRTR-91/06. U. S. Department of Interior, National Park Service, Denver, CO.
Shaffer M. 1968. Illustrated notes on Synclita obliteralis (Walker) and Euzophora bigella (Zeller), two species new to the British List (Lepidoptera: Pyralidae). Entomologist's Gazette 19: 155–158.
Stoops CA, Adler PH, McCreadie JW. 1998. Ecology of aquatic Lepidoptera (Crambidae: Nymphulinae) in South Carolina, USA. Hydrobiologia 379: 33–40.
Sutton DL. 1995. Hygrophila is replacing hydrilla in south Florida. Aquatics 17: 4, 6, 8, 10.
Tipping PW, Martin MR, Bauer L, Pierce RM, Center TD. 2012. Ecology of common salvinia, Salvinia minima Baker, in Southern Florida. Aquatic Botany 102: 23–27.
Williams FX. 1944. Biological studies in Hawaiian water-loving insects. Part IV. Lepidoptera or moths and butterflies. Proceedings of the Hawaiian Entomological Society 12: 180–185.
Woolfe T. 1995. Water weed is latest menace. Tallahassee Democrat, 4C, 3 August.
[USDA] U.S. Department of Agriculture. 2012. Federal Noxious Weeds List. USDA, Animal and Plant Health Inspection Service (APHIS), Plant Protection and Quarantine (PPQ) (23 April 2020).
Zimmerman EC. 1958. Volume 8 Lepidoptera: Pyraloidea. In Insects of Hawaii: a Manual of the Insects of the Hawaiian Islands, Including an Enumeration of the Species and Notes on their Origin, Distribution, Hosts, Parasites, etc. University of Hawaii Press, Honolulu. 456 pages.