Ultrasound has been used as a tool in beef and dairy research since the early 1980s (Perry and Cushman 2016). Since the early 2000s, it has become available to commercial livestock agriculture. An ultrasound is an electronic instrument that sends out ultrasonic sound waves from an attached device called a transducer. The waves pass freely through fluid and are reflected back to the probe once they contact a soft tissue like muscle or a dense structure like bone, resulting in an image that can be identified as the placenta, fetus, or other organs. Ultrasound may be used to determine subcutaneous fat content on finishing cattle (Hicks 2014); it is also an important diagnostic tool for veterinary medicine. More recently, Doppler ultrasonography has been used as a novel tool in cattle reproduction, because it estimates the blood flow to reproductive organs as a measure of their functionality. This publication aims to discuss the use of ultrasound to assist with beef cattle reproduction, which includes evaluation of the pre-service status of heifers and cows, diagnosis of pregnancy, determination of fetal age and sex, and evaluation of reproductive fitness of embryo recipients. This report is intended to be used by county Extension faculty in educating producers on reproductive management on cow-calf operations, and by producers who are interested in learning about the uses of a powerful technology to increase reproductive efficiency in their operations.
Reproductive Evaluation of Yearling Heifers prior to the Breeding Season
Yearling replacement heifers are critical to cow-calf operations. Reproductive success of replacement heifers depends on how close the heifers are to reaching puberty (Holm et al. 2009). Mature heifers become pregnant early in their first breeding season and remain longer in the maternal herd (Cushman et al. 2013). The reproductive maturity of heifers can be estimated by a method called “reproductive tract score” or RTS (Table 1) (Anderson et al. 1991), which varies from 1 to 5. Heifers with an RTS of 1 to 3 are called immature or prepubertal, meaning that they have not shown estrus yet and are not ready to be naturally or artificially serviced. On the other hand, heifers with an RTS of 4 or 5 are called mature or pubertal; they have shown estrus and are ready to be serviced. The methodology to evaluate RTS consists of making the following technical determinations: uterine size and tonus by rectal palpation, presence of a corpus luteum (CL), and size of the largest ovarian follicle by transrectal ultrasonography. According to these parameters (Table 1), heifers receive an RTS of 1 to 5.
Table 1. Reproductive tract score (RTS) system. Adapted from Anderson et al. (1991).
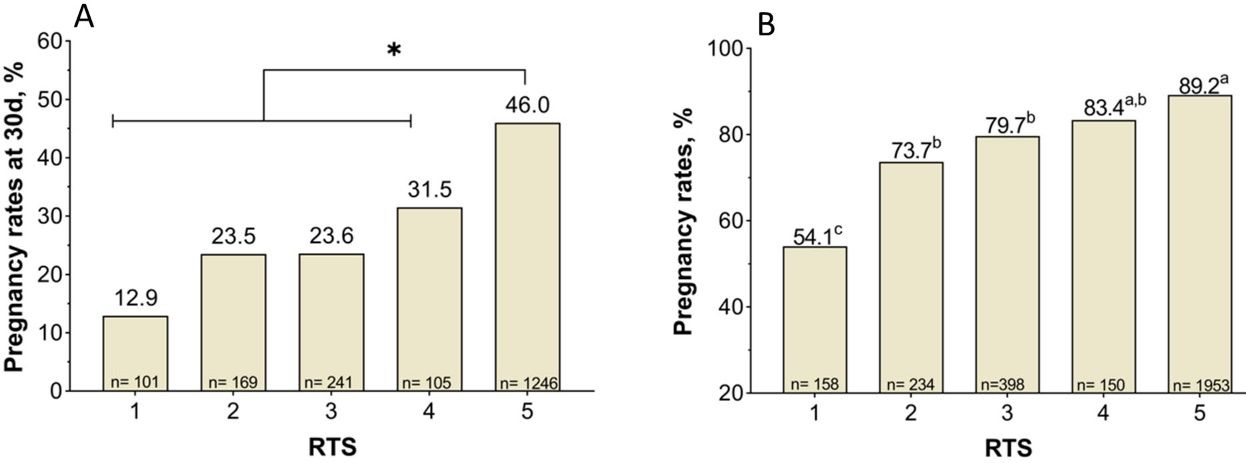
The RTS is associated positively with pregnancy rates attained during the breeding season (Holm et al. 2009). Indeed, RTS 5 heifers become pregnant earlier during the breeding season and present the greatest reproductive performance as first-calf heifers (Holm et al. 2009). Moreover, data collected in Florida confirmed that RTS 5 heifers presented the greatest pregnancy rates to artificial insemination (AI), measured at 30 days (Figure 1A), and at the end of the breeding season (Figure 1B). Thus, the evaluation of RTS before the beginning of the breeding season provides a management tool to make strategic decisions. For example, producers may opt to cull RTS 1 heifers, which will likely present the lowest pregnancy rates (e.g., <60%) and may not be cost effective to develop, depending on the production system. Culling a large proportion of heifers because they are RTS 1–3 may be burdensome. Another option is to induce puberty by using estrus synchronization programs (https://beefrepro.org/wp-content/uploads/2022/01/2022-Cow-and-Heifer-Protocols-for-Sire-Directories.pdf) based on intravaginal progesterone-releasing devices (such as CIDR) or oral progestins (such as MGA). In addition to benefiting the reproductive performance of mature heifers, the treatment of immature heifers with progesterone hastens puberty onset (Lucy et al. 2001). The long-term estrus synchronization programs seem to be the most favorable to improve reproductive performance of immature heifers.
Credit: Binelli Lab, UF/IFAS
Pregnancy Diagnosis and Resynchronization
Pregnancy diagnosis in cattle using ultrasound is an accurate, rapid, and safe method (Fricke and Lamb 2002; Baxter and Ward 1997). This is a non-invasive tool that can be used to diagnose pregnancy in cattle without harmful effects to either the fetus or the dam. At 30 to 40 days of pregnancy, Baxter and Ward (1997) did not report an increase in fetal losses when assessing pregnancy by ultrasound. Although ultrasound can be used for pregnancy diagnosis, after 45 days of pregnancy, there is not an increase in diagnostic accuracy when compared to rectal palpation performed by an experienced technician (Fricke and Lamb 2002). However, ultrasonography may improve diagnostic accuracy of technicians who are less experienced in rectal palpation (Fricke and Lamb 2002). The main advantage of using ultrasonography for pregnancy diagnosis is that it allows the detection of early gestations. Using the conventional brightness mode or B-mode makes it possible to detect the presence of a viable embryo as early as 28 days after mating (Ribadu et al. 1999). An ultrasound at 30 days can serve as a diagnostic tool after artificially inseminating the animals. For example, at this early diagnostic exam, a lower-than-expected proportion of females diagnosed as pregnant may indicate that semen quality, AI technique, and synchronization protocol should be investigated to decrease further financial burden. Additionally, pregnancy diagnosis 30 days after artificial insemination can allow producers to identify the pregnancies derived from artificial insemination and manage females differently than those pregnant by natural service.
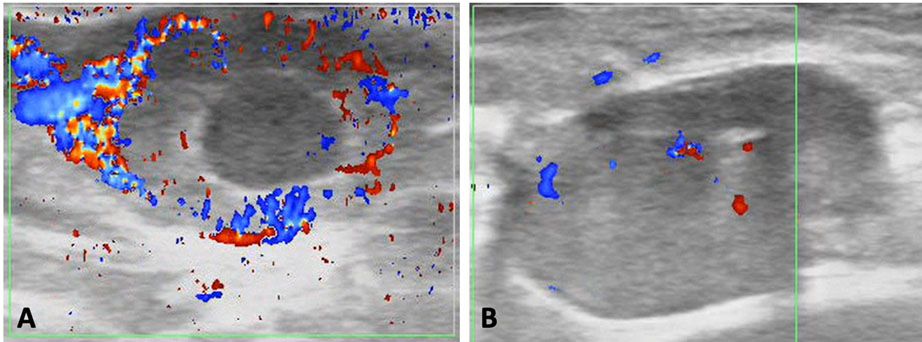
Ultrasonography has also been fundamental to the development and implementation of programs of estrus resynchronization. The adoption of estrus synchronization programs improves reproductive performance of a cow-calf enterprise (Rodgers et al. 2012). Among other advantages, it increases overall pregnancy rates and proportion of females becoming pregnant during the beginning of the breeding season (Rodgers et al. 2012). These two factors are important because they are associated with an increase in pounds of calf produced at weaning. The resynchronization further increases the likelihood of these beneficial effects (Bó et al. 2016; Baruselli et al. 2018; Ojeda-Rojas et al. 2021), favoring productivity. Currently, the conventional resynchronization programs consist of inserting CIDRs in all inseminated animals before knowing their pregnancy status. On day 28, CIDRs are removed and pregnancy diagnosis based on B-mode ultrasonography is performed in all cows. Pregnant cows are not handled further, while open females receive additional synchronization treatments and are subsequently re-inseminated. The super-early resynchronization is nowadays the state of the art on the topic of resynchronization (Baruselli et al. 2018; Ojeda-Rojas et al. 2021). Such programs allow repeated inseminations of females, every 24 days, which is very close to the physiological 21-day estrous cycle interval. The development of such a program was only possible because of the use of Doppler ultrasonography for pregnancy diagnosis (Pugliesi et al. 2014). By using this technology, the technician evaluates the blood flow to the corpus luteum as early as 20 days after AI. Through this ultrasonography exam, the pregnancy diagnosis shifts from the visualization of a fetus to the determination of a functional corpus luteum as a proxy of the pregnancy. A well-vascularized corpus luteum indicates an ongoing pregnancy with a 90% accuracy (Figure 2A), while a poorly-vascularized corpus luteum (Figure 2B) indicates that the female is not pregnant, with a 99.9% accuracy.
Credit: Gonella-Diaza Lab, UF/IFAS
Estimation of Age and Sex of a Fetus
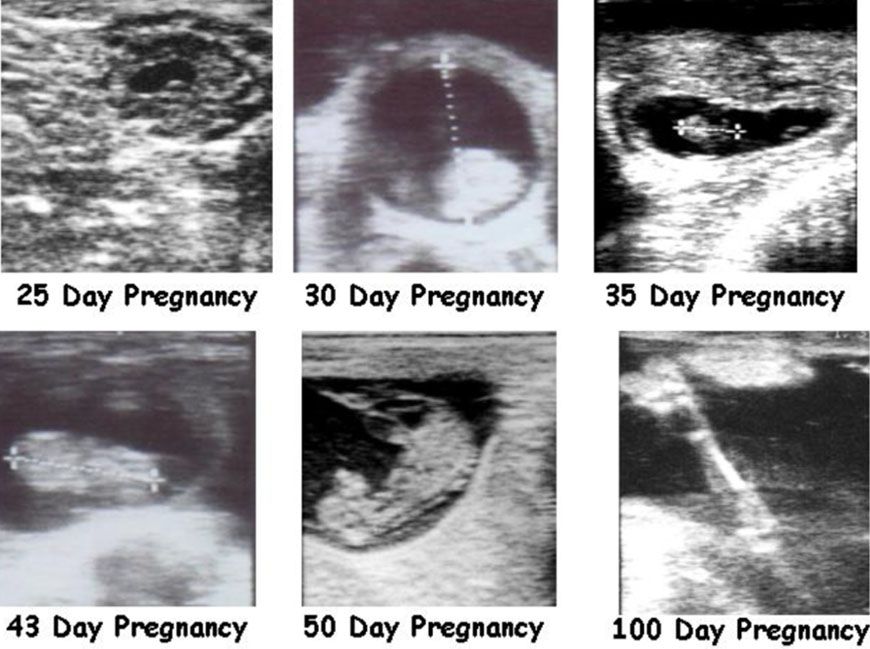
A trained technician can determine the pregnancy age based on the size of the embryo (30 to 45 days) or fetus (Figure 3). After 90 days of pregnancy, estimating pregnancy age might be difficult due to the position of the uterus in the abdominal cavity, the high liquid volume, and the large fetal size. To determine the pregnancy age, the technician uses specific embryo or fetal measurements such as crown-rump length (Table 2) (Hughes and Davies 1989). Estimated pregnancy age at the final pregnancy diagnosis can help producers to divide the herd into groups of females that became pregnant early or late during the breeding season. For example, late-pregnant cows may be maintained on pastures that will optimize calf growth to alleviate differences in calf weaning weights. Producers may wish to use creep feeding for calves born to cows in the late-pregnant group, or supplement these cows in a way that might lessen the following postpartum interval. Finally, producers may wish to wean these groups on different dates, optimizing calf uniformity and market price.
Credit: Adapted from Lamb (2001)
Table 2. Identification of fetal age by crown-rump length. Adapted from Curran et al. (1986), and Perry and Cushman (2016).
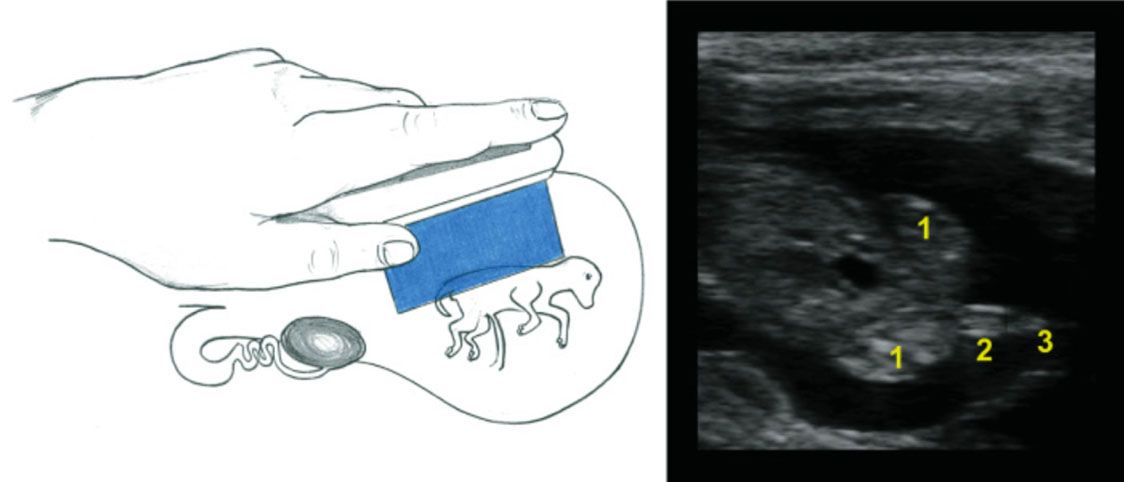
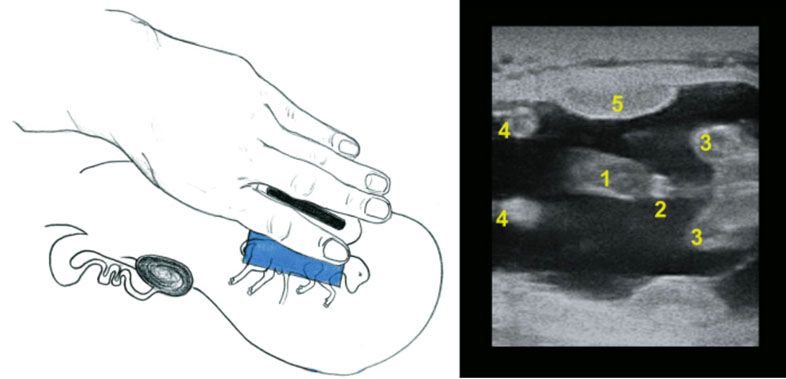
Another important function of an ultrasonography exam is to determine the fetal sex. Between 60 and 85 days of pregnancy, a trained technician can determine fetal sex, according to the localization of the genital tubercle with over 95% accuracy. For a female, it is located under the tail (Figure 4), while it is located slightly caudal to the umbilicus in a male (Figure 5). For more information about fetal sexing technique and images, refer to chapter 7 of Practical Atlas of Ruminant and Camelid Reproductive Ultrasonography (https://onlinelibrary.wiley.com/doi/book/10.1002/9781119265818). After 85 days of pregnancy, it may be difficult to obtain an adequate image for fetal sexing due to the increased size of the fetus and the enlarged and downward position of the uterus in the body cavity (Fricke and Lamb 2002). The determination of fetal sex can also help producers to manage pregnant cows in groups aiming to optimize determined progenies. An example of this management strategy is found in purebred operations that aim to separate cows giving birth to bull calves from those giving birth to heifers. Producers would then be able to divide their herds and manage each of the categories in a way that best fits their production system for heifer and bull calves. Fetal sexing using ultrasonography will become less important as sex-sorted semen becomes available and the sex of the offspring is selected with high accuracy prior to service (Holden and Butler 2018).
Credit: Adapted from Practical Atlas of Ruminant and Camelid Reproductive Ultrasonography (https://onlinelibrary.wiley.com/doi/book/10.1002/9781119265818)
Credit: Adapted from Practical Atlas of Ruminant and Camelid Reproductive Ultrasonography (https://onlinelibrary.wiley.com/doi/book/10.1002/9781119265818)
Evaluation of the Reproductive Fitness of an Embryo Recipient
Embryo transfer is an advanced reproductive technology meant to rapidly increase the genetic merit of a herd by transferring genetically superior embryos to fertile recipients. Another novel use of Doppler-mode ultrasonography is to evaluate the fitness of embryo recipients before embryo transfer (i.e., 7 days after estrus) (Pugliesi et al. 2019). Size and vascularization of the corpus luteum in embryo recipients are associated positively with pregnancy success for embryo transfer.
Additional Common Uses of Reproductive Ultrasound Technology in Florida
Ultrasound for yearling heifers 30 days after the end of the breeding season can be used to accurately (95% to 100%) determine pregnancy status compared to rectal palpation (which achieves the same accuracy only between 50 and 60 days after service). Producers are able to make immediate culling decisions on heifers, which reduces the feed cost significantly.
For large groups of yearling heifers, ultrasonography may assist in culling heifers based on fetal size. Only the heifers that become pregnant in the first 60 days of a 90-day breeding season would be retained. The remaining late-pregnant heifers would be sold and not kept in the cow herd. This allows producers to sell pregnant heifers that are worth more than open heifers.
For commercial producers who use AI programs, ultrasound may be used at 50 to 60 days after AI to identify which cows are pregnant from AI compared to natural service. In production systems that use timed AI programs, ultrasound can be performed as early as 30 days after AI, allowing producers to make management and strategic reproductive decisions during the breeding season. Identifying cows pregnant to AI (by notching ear tags or inserting an additional ear tag) allows producers to sort cows into calving groups the following spring based on whether they were pregnant to AI or not. Ultrasound allows producers to identify the cows that were not pregnant at the end of the breeding season and can therefore be culled earlier, which reduces feeding cost and increases profitability.
First-calf heifers are more prone to dystocia (calving problems). By identifying service dates, approximate calving dates can be determined. This directs increased observation of animals during the calving of first-calf heifers to help reduce the incidence of calf loss from calving difficulties.
There are other potential uses for ultrasound in cow-calf production systems. In Florida, the expansive nature of beef production systems generally does not afford cattlemen access to the cows when ultrasound may be most effective. Nonetheless, transrectal ultrasonography has and will continue to have a role in the successful reproductive management of cattle herds (Perry and Cushman 2016). There are many advantages to incorporating ultrasound into reproductive management practices for beef cattle. Contact your local UF/IFAS Extension agriculture agent to find a trained ultrasound technician in your area. If you need more information, contact your beef state specialist.
Acknowledgments
We thank the hard-working staff of the UF/IFAS Gainesville Beef Unit and students who collaborated with data collection and compilation. We also thank beef operations across Florida for allowing use of their cattle to conduct the research reported in this publication. We are grateful to Zoetis for the donation of drugs used for estrus synchronization, and to Estrotect for donating breeding indicators.
References
Anderson, K. J. D, D. G. LeFever, J. S. Brinks, and K. G. Odde. 1991. “The Use of Reproductive Tract Scoring in Beef Heifers.” Agri-Practice 12:19–26.
Baruselli, P. S., R. M. Ferreira, M. F. Sá Fillho, and G. A. Bó. 2018. “Using Artificial Insemination v. Natural Service in Beef Herds.” Animal. 12 (S1): s45–s52. https://doi:10.1017/S175173111800054X
Baxter, S. J., and W. R. Ward. 1997. “Incidence of Fetal Loss in Dairy Cattle after Pregnancy Diagnosis Using an Ultrasound Scanner.” Vet. Rec. 140:287–288. https://doi.org/10.1136/vr.140.11.287
Bó, G. A., J. V. de la Mata, P. S. Baruselli, and A. Menchaca. 2016. “Alternative Programs for Synchronizing Ovulation in Beef Cattle.” Theriogenology. 86:388–396. http://dx.doi.org/10.1016/j.theriogenology.2016.04.053
Curran, S., R. A. Pierson, and O. J. Ginther. 1986. "Ultrasonographic Appearance of the Bovine Conceptus from Days 20 through 60." J. Am. Vet. Med. Assoc. 189:1295–1302.
Cushman, R. A., L. K. Kill, R. N. Funston, E. M. Mousel, and G. A. Perry. 2013. “Heifer calving date positively influences calf weaning weights through six parturitions.” J. Anim. Sci. 91:4486–4491. https://doi.org/10.2527/jas2013-6465
Fricke, P. M., and G. C. Lamb. 2002. "Practical Applications of Ultrasound for Reproductive Management of Beef and Dairy Cattle." In The Applied Reproductive Strategies in Beef Cattle Workshop. Sep. 5–6, 2002. Manhattan, Kansas.
Hicks, C. 2014. “Using Live Animal Carcass Ultrasound in Beef Cattle.” University of Georgia Extension. Bulletin 1337. https://secure.caes.uga.edu/extension/publications/files/pdf/B%201337_3.PDF
Holden, S. A., and S. T. Butler. 2018. “Review: Applications and Benefits of Sexed Semen in Dairy and Beef Herds.” Animal 12:s97–s103. https://doi.org/https://doi.org/10.1017/S1751731118000721
Holm, D. E., P. N. Thompson, and P. C. Irons. 2009. “The Value of Reproductive Tract Scoring as a Predictor of Fertility and Production Outcomes in Beef Heifers.” J. Anim. Sci. 87:1934–1940. https://doi.org/10.2527/jas.2008-1579
Lamb, G. C. 2001. “Reproductive Real-Time Ultrasound Technology: An Application for Improving Calf Crop in Cattle Operations.” In Factors Affecting Calf Crop: Biotechnology of Reproduction, edited by M. J. Fields. 231–253. Boca Raton, FL: CRC Press.
Lucy, M. C., H. J. Billings, W. R. Butler, L. R. Ehnis, M. J. Fields, D. J. Kesler, J. E. Kinder, R. C. Mattos, R. E. Short, E. A. Hughes, and D. A. Davies. 1989. "Practical Uses of Ultrasound in Early Pregnancy in Cattle." Vet. Rec. 124:456–458.
Lucy, M. C., H. J. Billings, W. R. Butler, L. R. Ehnis, M. J. Fields, D. J. Kesler, J. E. Kinder, R. C. Mattos, R. E. Short, W. W. Thatcher, R. P. Wettemann, J. V. Yelich, and H. D. Hafs. 2001. “Efficacy of an Intravaginal Progesterone Insert and an Injection of PGF2α for Synchronizing Estrus and Shortening the Interval to Pregnancy in Postpartum Beef Cows, Peripubertal Beef Heifers, and Dairy Heifers.” J. Anim. Sci. 79:982–995. https://doi.org/10.2527/2001.794982x
Ojeda-Rojas, O. A., A. M. Gonella-Diaza, D. B. Bustos-Coral, G. L. Sartorello, T. S. S. S. Reijers, G. Pugliesi, M. E. Z. Mercadante, C. G. de Lima, and A. H. Gameiro. 2021. “An Agent-Based Simulation Model to Compare Different Reproductive Strategies in Cow-Calf Operations: Technical Performance.” Theriogenology 160:102–115. https://doi.org/10.1016/j.theriogenology.2020.10.035
Perry, G. A., and R. A. Cushman. 2016. "Invited Review: Use of Ultrasonography to Make Reproductive Management Decisions." Prof. Anim. Sci. 32:154–161.
Pugliesi, G., G. Dalmaso, D. Melo, J. Barboza, A. Sardinha, L. A. Silva, and M. Binelli. 2019. “Use of Color-Doppler Ultrasonography for Selection of Recipients in Timed-Embryo Transfer Programs in Beef Cattle.” Theriogenology 135:73–79. https://doi.org/10.1016/j.theriogenology.2019.06.006
Pugliesi, G., B. T. Miagawa, Y. N. Paiva, M. R. Franca, L. A. Silva, and M. Binelli. 2014. “Conceptus-Induced Changes in the Gene Expression of Blood Immune Cells and the Ultrasound-Accessed Luteal Function in Beef Cattle: How early can we detect pregnancy?” Biol. Reprod. 91:95. https://doi.org/10.1095/biolreprod.114.121525
Ribadu, A. Y., and T. Nakao. 1999. “Bovine Reproductive Ultrasonography: A Review.” J. Reprod. Dev. 45:13–28. https://doi.org/10.1262/jrd.45.13
Rodgers, J. C., S. L. Bird, J. E. Larson, N. DiLorenzo, C. R. Dahlen, A. DiCostanzo, and G. C. Lamb. 2012. “An Economic Evaluation of Estrus Synchronization and Timed Artificial Insemination in Suckled Beef Cows.” J. Anim. Sci. 90:4055–4062.