Introduction
What are the betanodaviruses?
The betanodaviruses are an important, emerging group of viruses known to infect over 40 marine fish species worldwide, including populations in Australia, Asia, Europe, North America, Africa, and the South Pacific (Walker and Winton 2010). Betanodavirus infections have also been reported to infect freshwater fish species in both natural and experimental populations.
As members of the family Nodaviridae, viruses in the genus Betanodavirus share a number of features that help scientists to distinguish them from other viruses. These features include a small, non-enveloped and icosahedral-shaped capsid that surrounds genetic material made up of single-stranded, positive-sense RNA. "Non-enveloped" means that these viruses lack an outermost layer made up of lipids and proteins, and the "capsid" is the protein "shell" that surrounds the genetic material of the virus. "Single-stranded, positive-sense RNA" identifies just the type of genetic material they contain.
The diseases caused by these viruses are commonly known as viral nervous necrosis (VNN) or viral encephalopathy and retinopathy (VER). These viruses damage the central nervous system in susceptible fish species and typically affect younger stages of fish (larvae, fry, fingerlings), although older, market-size fish can be affected as well, with losses ranging from 15–100%.
Currently, there are four species of betanodavirus (ICTV 2024) based on genetic analyses: a) Striped jack nervous necrosis virus (SJNNV); b) Barfin flounder nervous necrosis virus (BFNNV); c) Tiger puffer nervous necrosis virus (TPNNV); and d) Redspotted grouper nervous necrosis virus (RGNNV). It appears that each of these different species of betanodavirus may infect a number of other fish species; however, knowing which strain is infecting your fish does not change recommendations regarding prevention.
Within what temperature range can betanodaviruses infect fish and cause disease?
Betanodaviruses can infect tropical, sub-tropical, or cold-temperate species. Optimal temperature ranges for the betanodaviruses vary somewhat depending upon the strain of the virus and the species of fish. Optimal temperatures for SJNNV are 20–25°C (68–77°F); for BFNNV, 15–20°C (59–68°F); and for TPNNV, 20°C (68°F). For RGNNV, favorable temperature ranges are approximately 25–30°C (77–86°F) (Hata et al. 2007), with growth in cell culture optimal at 25°C (77°F) (Ciulli et al. 2006). Upper temperature limits for RGNNV appear to be ~32°C (90°F), based on laboratory studies (Hata et al. 2007).
Which fish species are susceptible?
Betanodaviruses are primarily a concern in marine species and have been reported in numerous species of marine food, game, and ornamental fishes. The following abridged lists are intended to show the diversity of susceptible species. Marine food and game fishes include red drum (Sciaenops ocellatus), cobia (Rachycentron canadum), sea bass (Dicentrachus labrax), barramundi (Lates calcarifer), gilthead seabream (Sparus aurata), Pacific bluefin tuna (Thunnus orientalis), various grouper species (Family Serranidae), and various flatfish species, including halibut (Hippoglossus hippoglossus) and Japanese flounder (Paralichthys oliveatus).
Marine ornamental fish species reported susceptible to betanodavirus infections include convict surgeonfish (Acanthurus triostegus), lined surgeonfish (A. lineatus), narrowstripe cardinalfish (Apogon exostigma), threespot dascyllus (Dascyllus trimaculatus), Scopas tang (Zebrasoma scopas), blueband goby (Valenciennea strigata), tiger puffer (Takifugu rubripes), lemonpeel angelfish (Centropyge flavissimus) (Bigarre et al. 2010) and the orbicularis batfish (Platax orbicularis) (David et al. 2010).
Susceptible freshwater species include tilapia (Oreochromis niloticus) (Bigarre et al. 2009) and the ornamental fish the guppy (Poecilia reticulata) (Hegde et al. 2003).
In laboratory studies, medaka (Oryzias latipes) were susceptible, developing clinical signs of disease after betanodavirus was administered by injection (adults) and immersion (larvae) (Furasawa et al. 2006). Experimentally infected zebrafish (Danio rerio) adults and larvae also developed disease signs after injection with virus (Lu et al. 2008).
What are typical external and internal signs of disease?
Betanodaviruses more commonly cause disease and mortality in larval and juvenile stages, but under the right conditions, sub-adults, market-size, and adult fish (including broodstock) can be affected. Clinical signs reflect the fact that the nervous system is targeted. In addition to mortalities of up to 100%, infected larvae and juvenile stages often show abnormal swimming behavior, including vertical positioning and spinning; flexing of the body; and muscle tremors. Betanodavirus causes hyperinflation of the swim bladder, so diseased fish are found primarily at the surface. Affected fish may also have traumatic lesions due to uncontrolled swimming/spinning. The most common clinical sign in adults is abnormal swimming (Bovo and Florio 2008). Changes in skin pigmentation, either darkening or lightening depending upon species, may also be seen.
Can betanodavirus infections be confused with any other diseases?
Yes, because changes in pigmentation and swimming behavior can be caused by other infectious agents (bacteria, virus, parasites) or environmental factors including water quality (e.g., high ammonia levels) or other toxins (e.g., pesticides).
How do I tell if my fish has a betanodavirus infection?
Special diagnostic tests are required, and therefore only a fish health professional or fish disease diagnostic laboratory can determine whether or not your fish has a betanodavirus infection. Appropriate diagnostic tests include histopathology, in which a preparation of thin, fixed-tissue sections are placed on a glass slide, stained, and examined under the microscope; virus isolation, which requires growing the virus in cell culture; testing for evidence of antibodies made by the fish against the virus; use of an electron microscope to look for viral particles themselves; and use of polymerase chain reactions (PCR) or DNA probes (pieces of genetic material that will bind specifically to betanodaviruses) in order to reveal evidence of viral DNA.
When a fish health professional or pathologist examines tissues prepared properly for histopathology, the most distinctive finding is the presence of vacuolation ("holes") (Figure 1) and necrosis (presence of dead tissue) in the brain, spinal cord, and retina of the eye. Inflammation may also be seen in the eye. However, in some cases histopathology may not be obvious.
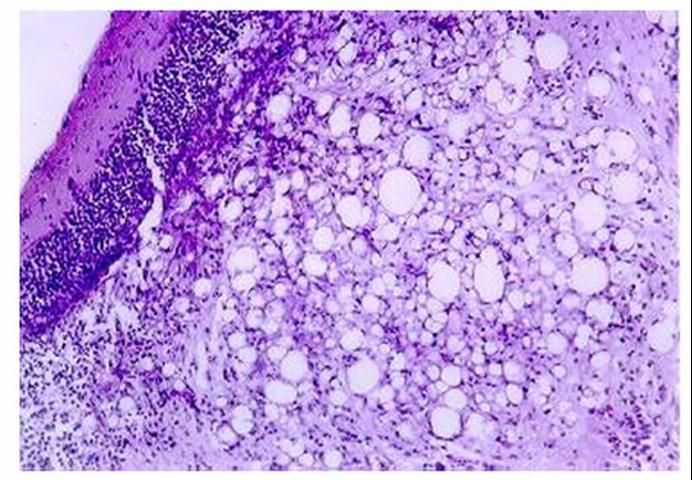
What factors contribute to development and spread of betanodavirus disease?
As with other viral diseases, environmental and procedural stressors, including high density and high temperatures, will weaken the immune systems of fish and make them more susceptible to infectious diseases including betanodavirus. Good husbandry may help reduce infection rates and losses. However, because many different factors often lead up to a single disease outbreak, a complete evaluation of the facility, including review of management protocols (including biosecurity measures) and environmental parameters, should be carried out. A review of water quality parameters, nutrition, and procedural stressors is especially important. In addition, a necropsy of a representative sample of fish that are showing clinical signs of disease should be performed to rule out other contributory causes.
Current studies suggest that betanodaviruses have the ability to infect numerous species of marine fish and some species of freshwater fish. Spread of the disease, therefore, may occur by introduction of infected fish into naïve populations. Infectivity may be increased if the immune systems of naïve fish are compromised because of environmental or other stressors. Older fish may also become more susceptible to disease during periods of increased temperatures.
Betanodaviruses may be found in a number of different reservoirs. Infected fish (wild or cultured) can act as a reservoir and may be a source for net pen aquaculture species. In one study, subclinical striped jack (Pseudocaranx dentex) broodstock ("carriers") were infected with betanodavirus in multiple organs including gonads and intestines, but not within the central nervous system (Nguyen et al. 1997). This suggests that these fish, which do not show signs of the disease, may spread the virus through their gametes (eggs or sperm) or feces. Another study demonstrated evidence of betanodaviruses (RGNNV) using PCR in the tissues of several species of wild marine invertebrates, including Charybdid crab (Charybdis bimaculata), southern humpback shrimp (Pandalus hypsinotus), and the Mediterranean mussel (Mytilus galloprovincialis) (Gomez et al. 2008). Additional research is needed to determine whether these animals may serve as true reservoirs and/or spread the virus to fish.
Laboratory studies have demonstrated that betanodavirus can be spread horizontally (from infected fish to uninfected, naïve fish in the same water body) or by exposure to contaminated water (water that contains virus). Betanodaviruses may also be transmitted through feeding of contaminated live foods, including Artemia (brine shrimp), Tigriopus japonicus (copepod), and Acetesinte medius (shrimp), or through feeding of raw contaminated fish. Trash fish species, such as Japanese jack mackerel (Trachurus japonicus), and mollusks, such as Japanese common squid (Todarodes pacificus), fed as food were determined to be a potential source of betanodavirus (RGNNV) (Gomez et al. 2010).
Finally, betanodaviruses have been shown to transmit vertically, i.e., from broodstock to offspring (Breuil et al. 2002; Kai et al. 2010), potentially within or on the outside of fertilized eggs.
Does betanodavirus infection cause a fatal disease?
Betanodavirus infections have been associated with significant mortality within populations, sometimes as high as 100%. Larvae and juveniles are typically affected, although older life stages can also succumb to infection. In addition, a number of clinically normal fish of different species have been tested using molecular methods (looking for viral RNA) and found to have evidence of betanodavirus infection (i.e., are subclinical or "carriers").
Can you treat fish with betanodavirus infections?
Currently there are no effective treatments for most viral infections of fish, including those caused by betanodavirus. Depopulation followed by disinfection is recommended.
What can I do to help prevent a betanodavirus outbreak?
Good husbandry and biosecurity are important, as is establishment of a good working relationship with a fish health professional and/or diagnostic laboratory. If you suspect you may have fish with a betanodavirus infection, sampling and special tests will be necessary. Contact a fish health professional for assistance.
Betanodavirus vaccines have been developed internationally and have been shown to reduce disease incidence and mortalities effectively in experimental trials. International vaccine studies have shown that vaccination of larvae and older grow-out fish can reduce disease incidence, and that vaccination of broodstock can reduce potential transmission of virus to offspring (Lin et al. 2007; Kai et al. 2010; Pakingking et al. 2010). Currently, no commercial vaccines are available in the United States.
For production facilities, washing and then disinfection of eggs after fertilization may help reduce infection from broodstock (if virus is on the outside of the egg). Use of ozone (at 1 mg O3/liter for one minute) for disinfection of embryos—after fertilization and water hardening—was found to be an effective control method for one species of fish (striped trumpeter, Latris lineata) (Battaglene and Morehead 2006). Laboratory tests by another research group with virus alone in water required significantly less ozone (see below).
Producers should also quarantine any new fish (and, if possible, invertebrates) in a separate building or area and follow appropriate biosecurity protocols (have dedicated equipment and use appropriate disinfectants) before adding them to existing farm populations. If possible, producers should also submit a representative sample of fish for additional testing, especially if any appear diseased or if mortalities occur.
Wholesalers and retailers should consider separating incoming fish groups by origin (e.g., imported vs. domestic, or by country of origin) and by species, to prevent or reduce likelihood of spread, but additional biosecurity measures are also important. Because betanodaviruses can be spread by contaminated water, use separate equipment (nets, siphon hoses) for each system, disinfect systems or tanks between uses, and place footbaths and hand disinfectants (alcohol spray or hand washing stations) judiciously. Isolate sick fish if possible, and handle them separately (see the UF Extension publication, "Fish Health Management Considerations in Recirculating Aquaculture Systems," parts 1–3: https://edis.ifas.ufl.edu/fa099, https://edis.ifas.ufl.edu/fa100, https://edis.ifas.ufl.edu/fa101).
Use of a water source that may have contained other fish or other potential sources of pathogens (e.g., creek, lake, or other "unprotected" water source) will increase risk as well. It is safer to use water from "protected" water sources, such as deep wells, or water that has been processed with adequate ultraviolet (UV) sterilization or chlorinated and dechlorinated (see below).
If I want to disinfect equipment after handling infected fish, what can I use?
Experimental studies have shown that betanodaviruses (SJNNV) can be inactivated with 50 mg/L sodium hypochlorite, calcium hypochlorite, benzalkonium chloride, or iodine for 10 minutes at 20°C (68°F); 60% ethanol; 50% methanol (no time given); pH of 12 for 10 minutes at 20°C (68°F); or heat at 60°C (140°F) for 30 minutes. The effective UV zap dose for inactivating BFNNV experimentally was 1.0 x 105 µ Wsec/cm2 at a UV intensity of 440 µ W/cm2 in one study and 1.5–2.5 x 105 µ Wsec/cm2 for inactivation of SJNNV in another. Ozone at 0.1 µ g/mL inactivated the virus (SJNNV) after 2.5 minutes (Arimoto 1996; Kasai et al. 2002). Higher doses for each of these methods may be required depending upon organic levels, turbidity, or other differences in water quality or chemistry.
Summary
Viral nervous necrosis (VNN), also known as viral encephalopathy and retinopathy (VER), is an important, emerging group of fish diseases caused by the betanodaviruses. Numerous marine finfish and some freshwater species worldwide have been reported to be susceptible to the betanodaviruses, with mortalities as high as 100%. In many reported cases, younger life stages are affected, although older fish are also susceptible.
Because these viruses target the central nervous system (including the brain, spinal cord, and eye), abnormal behavior (e.g., spinning or whirling, abnormal posture, muscle tremors) are the most common clinical signs. Other signs include darkening, traumatic lesions caused by swimming into structures, hyperinflation of the swim bladder in younger stages, and moderate to high mortalities. Since these signs can also be seen with other diseases, it is important to work closely with a fish health professional and/or fish disease diagnostic laboratory to properly identify the cause(s) of any disease problem.
Betanodaviruses can be spread from infected diseased fish, wild invertebrates, contaminated live or frozen foods, contaminated water, and carrier (subclinical) broodstock. Biosecurity measures for producers should include use of protected water sources, or adequate sterilization of source water; testing of potential broodstock and live or frozen foods; disinfection of fertilized eggs; and sampling and testing of incoming or diseased fish. Wholesalers and retailers should consider system separation of fish groups by origin (imported vs. domestic; importer by country; species).
Footbaths, dedicated equipment, and disinfection stations, as well as use of UV sterilization for recirculating systems will also help reduce potential spread.
References and Suggested Reading
Arimoto, M., J. Sato, K. Maruyama, G. Mimura, and I. Furusawa. 1996. Effect of chemical and physical treatments on the inactivation of striped jack nervous necrosis virus (SJNNV). Aquaculture 143:15–22.
Battaglene, S. C., and D. T. Morehead. 2006. Tolerance of striped trumpeter Latris lineata embryos to ozonated seawater. Aquaculture International 14:421–429.
Bigarre, L., J. Cabon, M. Baud, M. Heimann, A. Body, F. Lieffrig, and J. Castric. 2009. Outbreak of a betanodavirus infection in tilapia, Oreochromis niloticus (L.), in fresh water. Journal of Fish Diseases 32:667–673.
Bovo, G., and D. Florio. 2008. Chapter 4. Viral diseases of cultured marine fish. In Fish Diseases, vol. 1, Eiras, J.C., H. Segner, T. Wahli, and B. G. Kapoor, eds. Science Publishers, Enfield, NH. Pp. 202–216.
Breuil, G., J. F. P. Pepin, S. Boscher, and R. Thiery. 2002. Experimental vertical transmission of nodavirus from broodfish to eggs and larvae of the sea bass, Dicentrarchus labrax. Journal of Fish Diseases 25:697–702.
Ciulli, S., D. Gallardi, A. Scagliarini, M. Battilani, R. P. Hedrick, and S. Prosperi. 2006. Temperature-dependency of betanodavirus infection in SSN-1 cell line. Diseases of Aquatic Organisms 68(3):261–265.
Curtis, P. A., M. Drawbridge, T. Iwamoto, T. Nakai, R. P. Hedrick, and A. P. Gendron. 2001. Nodavirus infection of juvenile white seabass, Atractoscion nobilis, cultured in southern California: first record of viral nervous necrosis (VNN) in North America. Journal of Fish Diseases 24:263–271.
David, R., C. Tréguier, C. Montagnani, C. Belliard, P. Levy, G. Nédélec, V. Joufoques, G. Remoissenet, Y. Gueguen, and N. Cochennec-Laureau. 2010. Molecular detection of betanodavirus from the farmed fish, Platax orbicularis (Forsskal) (Ephippidae), in French Polynesia. Journal of Fish Diseases 33 (5): 451–454.
Furasawa, R., Y. Okinaka, and T. Nakai. 2006. Betanodavirus infection in the freshwater model fish medaka (Oryzias latipes). Journal of General Virology 87:2333–2339.
Gomez, D. K., G. W. Baeck, J. H. Kim, C. H. Choresca, Jr., and S. C. Park. 2008. Molecular detection of betanodaviruses from apparently healthy wild marine invertebrates. Journal of Invertebrate Pathology 97: 197–202.
Gomez, D. K., G. W. Baeck, J. H. Kim, C. H. Choresca, Jr., and S. C. Park. 2008. Molecular detection of betanodavirus in wild marine fish populations in Korea. Journal of Veterinary Diagnostic Investigation 20:38–44.
Gomez, D. K., K. Mori, Y. Okinaka, T. Nakai, and S. C. Park. 2010. Trash fish can be a source of betanodaviruses for cultured marine fish. Aquaculture 302:158–163.
Hata, N., Y. Okinaka, T. Sakamoto, T. Iwamoto, and T. Nakai. 2007. Upper limits for the multiplication of betanodaviruses. Fish Pathology 42(4):225–228.
Hegde, A., H. C. The, T. J. Lam, and Y. M. Sin. 2003. Nodavirus infection in freshwater ornamental fish, guppy, Poicelia (sic) reticulata—comparative characterization and pathogenicity studies. Archives of Virology 148:575–586.
ICTV (International Committee on Taxonomy of Viruses) online (Accessed February 5, 2024). https://ictv.global/report/chapter/nodaviridae/nodaviridae/betanodavirus
Kai, Y., H. Su, J. Tai, and S. Chi. 2010. Vaccination of grouper broodfish (Epinephelus tukula) reduces the risk of vertical transmission by nervous necrosis virus. Vaccine 28: 996–1001.
Kasai, H., M. Yoshimizu, and Y. Ezura. 2002. Disinfection of water for aquaculture. Proceedings of International Commemorative Symposium 70th Anniversary of the Japanese Society of Fisheries Science 68 (Supplement 1): 821–824.
Lu, M., Y. Chao, T. Guo, N. Santi, O. Evensen, S. K. Kasani, J. Hong, and J. Wu. 2008. The interferon response is involved in nervous necrosis virus acute and persistent infection in zebrafish model. Molecular Immunology 45: 1146–1152.
Munday, B. L., J. Kwang, and N. Moody. 2002. Betanodavirus infections of teleost fish: a review. Journal of Fish Diseases 25:127–142.
Nguyen, H. D., K. Mushiake, T. Nakai, and K. Muroga. 1997. Tissue distribution of striped jack nervous necrosis virus (SJNNV) in adult striped jack. Diseases of Aquatic Organisms 28:87–91.
Pakingking, Jr., R., N. B. Bautista, E. G. de Jesus-Ayson, and O. Reyes. 2010. Protective immunity against viral nervous necrosis (VNN) in brown-marbled grouper (Epinephelus fuscogutattus) following vaccination with inactivated betanodavirus. Fish and Shellfish Immunology 28: 525–533.
Petty, B. D., and W. Fraser. 2005. Viruses of pet fish, In Veterinary Clinics of North America: Exotic Animal Practice, Special Issue: Virology 8(1): 67–84. Saunders.
Thiery, R., J. Cozien, C. de Boisseson, S. Kerbart-Boscher, and L. Nevarez. 2004. Genomic classification of new betanodavirus isolates by phylogenetic analysis of the coat protein gene suggests a low host-fish species specificity. Journal of General Virology 85:3079–3087.
Walker, P. J., and J. R. Winton. 2010. Emerging viral diseases of fish and shrimp. Veterinary Research 41:51.
Yanong, R. P. E. 2009. Fish health management considerations in recirculating aquaculture systems—Parts 1–3. FA120–122. Gainesville: University of Florida Institute of Food and Agricultural Sciences. Accessed November 14, 2010. Available on line at: https://edis.ifas.ufl.edu/fa099; https://edis.ifas.ufl.edu/fa100; https://edis.ifas.ufl.edu/fa101