Introduction
Recirculating aquaculture systems, also known as water reuse systems, have become more and more popular.Recirculating systems are commonly found in aquaculture facilities, wholesale and retail tropical fish facilities, and public aquaria. However, in order to successfully and most efficiently operate one of these systems, a good understanding of fish health management considerations is critical.
This circular is Part 2 in a three-part series dealing with management of fish health in recirculating systems. It explains the role of pathogens (potential disease-causing organisms) in these systems. Part 1 introduces preventative medicine principles and explains purpose-built recirculating systems for different uses. Part 3 of this series gives some general recommendations and problem-solving approaches for management of recirculating systems. In combination, the three circulars provide basic information that should assist beginning or intermediate-level aquaculturists in maintaining healthy fish in recirculating systems. This series is a starting point for anyone considering installing or currently using a recirculating system.
Pathogens in Recirculating Systems
Water quality can be more unstable in recirculating systems than in large ponds or flow-through systems. Water quality fluctuations, such as temporary increases in ammonia or nitrite, can, by themselves, result in disease or significant losses. These environmental fluctuations often lead to suppressed immune systems and greater susceptibility to pathogens (i.e., disease-causing organisms, such as bacteria, parasites, fungi, and viruses) and disease outbreaks.
Recirculating systems favor the growth of many disease-causing organisms and spread of disease. There are a number of reasons for this tendency, including higher densities of fish when compared to other culture systems; build up of biofilms and sediment and subsequently pathogens in tanks, sumps, or filtration components (especially mechanical and biological filters); and slower turn over of water.
Over time, pathogens can become concentrated (i.e., present in high numbers). Most pathogens are considered opportunistic, causing disease only in fish with suppressed immune systems. However, if pathogens become sufficiently numerous they can also cause disease in healthy fish. In addition, the continuous flow of water throughout a system can spread pathogens rapidly, especially in a system lacking adequate disinfection protocols or components, such as ultraviolet sterilization or ozone (see System Disinfection below).
Bacteria, parasites, fungi and viruses can all become concentrated in recirculating systems. Bacteria that seem to increase in number in recirculating systems include Aeromonas spp., Vibrio spp., Mycobacterium spp., Streptococcus spp., and Flavobacterium columnare (Columnaris disease) (see UF/IFAS Fact Sheets FA-14 Aeromonas Infections, FA-31 Vibrio Infections of Fish and VM-96 Mycobacteriosis in Fish; UF/IFAS Circular 57 Streptococcal Infections of Fish; and SRAC Publication No. 479b Columnaris Disease, respectively). Parasites that tend to thrive and spread relatively easily in recirculating systems include Trichodina, Ichthyophthirius,Cryptocaryon, Amyloodinium, Costia and monogeneans (see UF/IFAS Circulars 716 Introduction to Freshwater Fish Parasites and 920 Ichthyophthirius multifiliis (White Spot) Infections in Fish; UF/IFAS Fact sheet Amyloodinium Infections in Fish VM-90;and UF/IFAS Fact Sheet FA-28 Monogenean Parasites of Fish, respectively). Closed systems can also foster the spread of fungi and viruses (see UF/IFAS Fact Sheets VM-97 Fungal Diseases of Fish and FA-29 Introduction to Viral Diseases of Fish, respectively).
Adequate control of pathogens in a system, and consequently reduction of disease in these systems, requires an understanding of where pathogens may be found, how they can be transmitted to fish, and how their numbers may be reduced. In addition, understanding the proper use of chemicals to reduce or eliminate pathogens is an essential part of good management.
Biosecurity
Biosecurity has been mentioned in Part 1 of this series (recommended reading), but its importance warrants further discussion. The purpose of a biosecurity program is to prevent entry of specific pathogens (disease-causing organisms, i.e., bacteria, viruses, parasites, or fungi) that may cause significant disease and are not present either in the environment or on the fish in a given facility or system. In some cases, this is achieved by extensive testing of fish prior to receiving them from a supplier, or during isolation and quarantine, prior to placing them in their intended system.
For some pathogens, this may not be an absolute elimination of risk of entry, but primarily an overall reduction of the number that do enter the facility, so that fish already on the property do not receive an overwhelming load.
Biosecurity measures are important not only when bringing new fish into a facility; these measures are also important for reducing overall numbers of potential pathogens in a given system, and to avoid transferring pathogens from one system to another. For this reason, it is important to understand where pathogens may be found (reservoirs), and why quarantine, disinfection, and sanitation are important to a good biosecurity program.
Pathogen Reservoirs
There are many areas within an aquaculture facility and recirculating system that can act as reservoirs for pathogens. The most important reservoirs are the fish themselves. Fish can act as asymptomatic carriers of disease. In other words, they may be immune to a specific pathogen but still be able to shed the organism into the water or transfer it to other fish by contact. Sick and dead fish are often major reservoirs of disease-causing organisms. For this reason, sick, moribund (dying), and dead fish should be removed as soon as possible from a system and disposed of according to county, state, or federal regulations. In most instances, disposal can be as simple as placing the dead fish in a plastic bag and putting it in a trash receptacle. Water can also act as a reservoir. Water can spread pathogens to anything it touches.
The ground (e.g., concrete slab) can contain pockets of water that contains pathogens. Equipment, including nets, siphon hoses and buckets, can also contain pockets of disease-causing organisms. For this reason, disinfection of floors, and use of footbaths (either small containers or mats containing disinfectants) placed at entrances and exits to system rooms is recommended, as is disinfection of all equipment when used with fish in different tanks or vats or systems. Nets should be kept off the floor and placed in an appropriate clean location to avoid contamination.
Quaternary ammonium compounds are commonly used to disinfect equipment but they must be rinsed adequately prior to reuse because these compounds are toxic to fish (see UF/IFAS Fact Sheet VM-87, Sanitation Practices for Aquaculture Facilities). Chlorine can be used but will destroy nets and must be neutralized or rinsed off adequately to avoid killing fish. Equipment disinfected with iodine-containing compounds must also be rinsed off prior to use because they can be toxic. Virkon Aquatic ® is used by numerous aquaculture facilities and has been shown to be safe and effective against a wide variety of aquatic pathogens when used as directed. Contact a fish health or aquaculture specialist for recommendations on disinfectants for equipment, floors, and footbaths.
System hardware, including sumps and filters, sediment, and tank walls, are common sites for pathogens. Sumps and tanks often contain a fine film (biofilm) or layer of sediment that may harbor pathogenic organisms. Sediment on the bottom of sumps and tanks should be vacuumed routinely. Uneaten food lying on the bottom of tanks can also provide areas for pathogens to flourish.
Filter beds, because of their particulate nature, concentrate microorganisms. Mechanical filters should be backwashed, as frequently as possible, to reduce the loads of the undesirable (non-biofilter) bacteria, as well as other potential pathogens.
Pathogen Transmission
Pathogens can be transmitted several ways within a recirculating system:
-
in the water
-
fish to fish
-
by vectors and fomites
-
in the food
Introduction of water used to ship fish can be a key source of pathogens. Shipping water often contains high numbers of bacteria and may also contain parasites or other pathogens. These organisms are easily transferred from tank to tank in the recirculating water, or by aerosolization (in mist or spray) of water from one tank or system to another.
Within a single tank or vat, pathogens can be spread directly from fish to fish. Higher stocking densities and increased fish-to-fish contact (as seen in aggressive species) can increase the rate of spread of pathogens.
Vectors are organisms that can transmit disease-causing organisms from one animal to another. For example, the crustacean parasite Argulus ("fish louse") causes damage by itself, but it is also believed to transmit bacteria and viruses between fish. Leeches are another vector that can transmit blood-borne parasites and bacteria between fish. Additionally, people can act as vectors by transmitting water and pathogens from one tank to another via their hands or arms.
Fomites are inanimate objects that can transmit diseases. Examples of fomites in aquaculture systems include equipment, such as nets and siphon hoses, that are not properly disinfected before being used in other tanks or vats.
Food can also be a source of disease. Frozen and live foods can transmit bacteria, parasites, viruses, and fungi. In addition, feeds that have been improperly stored can contain pathogenic bacteria or mycotoxins, dangerous chemicals produced by the growth of certain types of fungi in the feed (see UF/IFAS Fact Sheet FA-95, Molds in Fish Feeds and Aflatoxicosis). Improperly stored feeds also have reduced nutritional value, due to degradation of micronutrients (e.g., reduction of vitamin C levels) and macronutrients (e.g., rancidity of fats).
System Disinfection or Sterilization
As described previously, water may spread pathogens and also be a potential reservoir for them. Water from a tank containing sick fish often carries numerous disease-causing microorganisms. When this same water enters another tank of fish, those fish are then exposed to the microorganisms and they will have an increased risk of developing disease. Disinfection helps to greatly reduce the spread of some pathogens. Two techniques commonly used to disinfect water in aquaculture systems are ultraviolet sterilization and ozonation.
Ultraviolet Sterilization
Ultraviolet (UV) sterilizers typically consist of UV-producing lamps encased in a glass or quartz sleeve. Water is passed over the lamps. The lamps emit ultraviolet light (a wavelength of approximately 254 nm is considered optimal) that penetrates cells and damages genetic material (DNA and RNA) and proteins.
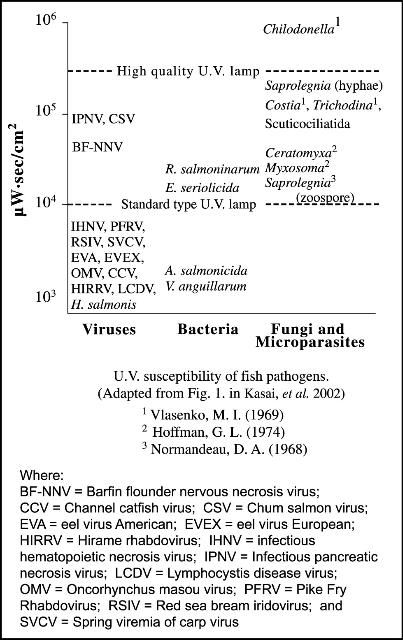
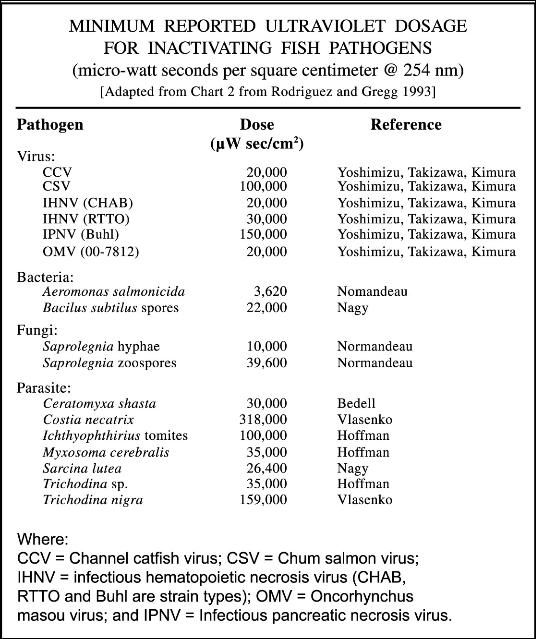
For each type of microorganism, a specific "zap dose," measured in microwatt seconds per square centimeter, is required to selectively sterilize the system (i.e., kill the unwanted organism). The zap dose is determined by the intensity or wattage of the lamp, contact time or flow rate of the water, water clarity, and size and biological characteristics of the target organism. In general, larger organisms require a larger zap dose (see Table 1 and Figure 1); however, the specific structure of certain viruses (which are generally much smaller than bacteria) makes some of them more difficult to "kill" than other larger organisms. In general, the zap dose required is lowest for gram-negative bacteria, and it increases progressively for gram-positive bacteria, viruses, spore-forming bacteria, and protozoans.
Ozonation
Ozone-disinfection systems introduce ozone, O3, a highly reactive molecule, into a contact chamber (isolated from the main system holding the fish). Ozone generators are more complex than UV-sterilizing units, and they require the presence of a high energy field through which dry filtered air or pure oxygen flows. The ozone oxidizes (i.e., reacts with and breaks down) dissolved and suspended molecules, as well as molecules within and on pathogens in the water. In freshwater systems, ozone rapidly breaks down or dissipates once it makes contact with the water; therefore, water from a contact chamber can be reintroduced into the system quickly, if the system is designed properly. However, as ozone is so highly reactive, all ozone must be eliminated from the water prior to its reintroduction. This elimination can be accomplished in several ways including off-gassing or removal by carbon filtration. Consult with an aquaculture specialist or a manufacturer of ozone sterilizers before purchasing a unit.
Ozone does not appreciably oxidize ammonia (i.e., convert ammonia into nitrite). In recirculating systems, this reaction is commonly accomplished by nitrifying bacteria in the biofilter. Ozone does oxidize nitrite to nitrate, so it augments the efforts of nitrifying bacteria in the biofilter. If the ozone is turned off in a system adapted to its presence, the nitrifying bacteria in the biofilter may not be present in high enough numbers to prevent nitrite levels from temporarily spiking in the system.
In addition to sterilizing water, ozone helps other parts of the system. Ozone promotes water clarity by rapidly breaking down dissolved and particulate organics that discolor or cloud the water. Ozone improves biofiltration efficiency by decreasing the organic load in the biofilter. This organic load is a food source for bacteria known as heterotrophs. Heterotrophs include many disease-causing bacteria that often compete with nitrifying bacteria for space and oxygen in the biofilter.
Ozone is more dangerous than UV sterilization. Small amounts in the water can kill fish and residual amounts in the air can be toxic to humans. In seawater, removal or dissipation of ozone is typically slower than in freshwater, and by-products of ozonation can increase the risk of disease in fish. For example, chemical by-products of ozonation have been suggested as one potential cause of head and lateral line erosion, although, research to demonstrate this association has not be completed. Also, some species are much more sensitive to residual ozone levels than others. There are different ways to monitor ozone levels in the water holding the fish. Consult a specialist for details on proper and safe use of ozone.
An aquaculture specialist or manufacturer of UV-sterilizers can provide advice about the size of sterilizer required for a specific system and specific pathogens. Maintenance and regular bulb replacement are important, because UV-bulbs quickly lose their initial strength. Most UV bulbs need replacing every 6–9 months.
Bacterial counts, run on agar plates, can be used to determine the effectiveness of UV sterilizers against bacteria in the water. More user-friendly kits are also available from some companies for bacterial count determination. Consult a fish health specialist or aquatic microbiologist for assistance.
Effects of Chemicals on the Biofilter
If changes in management, such as improvements in water quality, handling, or nutrition, cannot resolve a disease outbreak, chemicals may be required. Water changes are always recommended prior to placing tanks or vats containing the treated fish back on-line with the rest of the system.
Several studies, conducted in the late 1970s using certain chemicals, had different reported effects on biofilter capacity (Collins et al 1975; Collins et al 1976; Levine and Meade 1976; Spotte 1979). In general, fish health specialists DO NOT recommend using antibiotics as a bath treatment in a recirculating system.
Use of Antibiotics and Antibacterials
For species of food fish with specific, FDA-approved antibiotics, these antibiotics must be used according to FDA regulations. The chemical malachite green is explicitly illegal for use in food fish, as are other chemicals. If in doubt, contact a fish health specialist with regard to legal use of treatments for the species you are raising.
Diagnosis of a bacterial disease should be verified by a fish health specialist, and appropriate bacterial tests should be run to determine which antibiotic will be effective. Ideally, in ornamental fish, this antibiotic should be given in a medicated food, although in some cases injections may be warranted for valuable individuals.
In the ornamental fish industry and in public aquaria, antibiotic bath treatments are used, under strict control, when fish are not taking food or have external infections (see UF/IFAS Circular 84 Use of Antibiotics in Ornamental Fish Aquaculture). There are no FDA-approved antibiotics for use in all ornamental fish. The FDA has exercised regulatory discretion with regard to use of antibiotics in the ornamental fish industry. This means that currently they will not necessarily take legal action, unless a violation is considered worthy of such action. This situation may change in the future.
Under certain conditions, veterinarians have the legal right to prescribe antibiotics to their clients to be used in an "extra-label" manner. "Extra-label" means using the antibiotic in a way that is different from that for which it is specifically labeled. Therefore, aquaculturists are encouraged to work closely with a fish veterinarian during disease outbreaks as well as during development of a fish health management program.
Therefore, antibiotics should only be used in a bath under the following conditions:
-
the fish will not consume a medicated feed
-
in consultation with a fish veterinarian or fish health specialist
-
proper culture and sensitivity tests have been run to determine which antibiotic should be used
-
the treated fish are "taken off line", so the water is contained and will not contact the biological filter
-
the treated water is disposed of according to local regulations
Studies (Collins et al 1976; Levine and Meade 1976; Spotte 1979) have demonstrated that use of antibiotics as a bath treatment will negatively impact the biofilter, reducing its ability to function by as much as 44–100%.
Use of Other Chemicals
Research has shown that there are some differences in the inhibitory effects of formalin, malachite green (illegal for use in food fish), methylene blue (methylene blue is not recommended by fish health specialists and is illegal for use with food fish), copper sulfate, and potassium permanganate on the biofilter bacteria (Collins et al 1975; Levine and Meade 1976).
Different studies show different effects. Formalin used in one study, at 25 mg/L had no effect, whereas another study showed reduction of biofilter bacterial activity by 27% when used at 15 mg/L. As a rule of thumb, most aquaculturists do not consider use of formalin at 15–25 mg/L to have a major impact on the biofilter. However, when testing for ammonia levels, formalin will react with Nesslers Reagent (a component of most ammonia test kits) and can give a falsely elevated ammonia reading. In systems treated with formalin, the salicylate reagent test for ammonia is recommended (Hach Company 2002) because it does not react with aldehydes (e.g., formaldehyde found in formalin).
Malachite green (again, illegal for use in food fish) has been shown to have no effect on the biofilter at 0.1 mg/L, combined with or without formalin at 25 mg/L. Copper sulfate at 1 and 5 mg/L likewise had no effect on biofiltration.
By contrast, potassium permanganate experiments have been mixed. In one study, a 4-mg/L dosage resulted in no inhibition of the biofilter, whereas in another study, a 1-mg/L dosage resulted in an 86% inhibition.
The actual impact on an individual system will most likely depend upon many factors, such as chemical concentration, length of time in treatment, organic load, pH, temperature, alkalinity, filtration, oxygen levels, and stocking density; and, although this will most likely be true for most chemicals, this may better explain the differences in effect by potassium permanganate.
More work has to be conducted on the use of hydrogen peroxide in recirculating aquaculture systems, especially its safety and efficacy for use in various fish species and system configurations, including effects on biofiltration. More organics in the system lessen the likelihood that biofilter bacteria will be damaged or killed by these chemicals. However, too high an organic load will render these chemicals ineffective as treatments.
If possible, affected fish should be treated in vats or tanks that have been taken off-line from the remainder of the system, and a 75–100% water change should be done in those vats or tanks prior to their being placed back on-line.
It is important to reiterate that antibiotics are NEVER recommended for use in system-wide bath treatments because of the potential for development of antibiotic-resistant strains of pathogens and severe detrimental effects on the nitrifying bacteria within the biofilter. If a population of fish in a recirculating system must undergo a specific antibiotic bath treatment, that population should be isolated from the rest of the system by shutting off water flow during treatment. After treatment, 100% water changes for the treated vats or tanks are recommended.
Salt (sodium chloride)
Another chemical commonly used in recirculating systems is salt (sodium chloride). Salt can be useful in reducing certain parasite infections in a system. Salt also helps reduce osmolarity stresses by increasing the salt concentration in the water relative to the normal concentration in the fish's body (freshwater fish have a higher body salt content than freshwater and so must use energy to keep the body's salt concentration—the osmolarity—in balance). Most tropical fish can tolerate a salt concentration of 1–3 g/L, and this level is not harmful to the biological filter.
Summary
Before new fish are placed into a new system, or into a system already containing fish, biosecurity protocols (including quarantine, sanitation, and disinfection) should be followed.
Recirculating systems may promote the growth of certain disease-causing organisms (pathogens). A good understanding of where these pathogens (parasites, bacteria, fungi, and viruses) may exist in a system and how they may enter a system is important for the recirculating system manager. This understanding is critical for good system design and for the development of effective management protocols.
Pathogens can be found on fish in the system; in the water; on system hardware including the facility floor; on surfaces of tanks, sumps, and filter beds; and on husbandry equipment such as nets and siphons. They can be transmitted by water, from fish to fish, by vectors (other organisms including people), by fomites (non-living things such as nets), and by contaminated feed.
Ultraviolet and ozone sterilizing units can help reduce overall pathogen numbers in a system, but they will not prevent spread of pathogens within a system unit (e.g., tank or vat). Sterilizing units must be sized properly, according to manufacturer's recommendations, and they also must be strictly maintained. Only the water that contacts the sterilizers will be affected. Poor husbandry (for example, not dipping nets when using them between tanks) will negate any benefits of these sterilizing systems. Also, ozone can be dangerous to fish and humans.
Chemicals used in a system may have undesirable effects on the water, biological filter, the fish, or employees. Therefore the pros and cons of each chemical used in the system must be understood. All chemicals should only be used in an appropriate manner.
Recommended Reading
SRAC Publication No. 479b Columnaris Disease. https://srac.tamu.edu/index.cfm/event/getFactSheet/whichfactsheet/128/
UF/IFAS Circular 57 Streptococcal Infections of Fish. https://edis.ifas.ufl.edu/FA057
UF/IFAS Circular 84 Use of Antibiotics in Ornamental Fish Aquaculture. https://edis.ifas.ufl.edu/FA084
UF/IFAS Circular 120 Fish Health Management Considerations in Recirculating Aquaculture Systems—Part 1: Introduction and General Principles. https://edis.ifas.ufl.edu/FA099
UF/IFAS Circular 122 Fish Health Management Considerations in Recirculating Aquaculture Systems—Part 3: General Recommendations and Problem-Solving Approaches. https://edis.ifas.ufl.edu/FA101
UF/IFAS Circular 716 Introduction to Freshwater Fish Parasites. https://edis.ifas.ufl.edu/FA041.
UF/IFAS Circular 919 Stress—Its Role in Fish Disease. https://edis.ifas.ufl.edu/FA005.
UF/IFAS Circular 920 Ichthyophthirius multifiliis (White Spot) Infections in Fish. https://edis.ifas.ufl.edu/FA006.
UF/IFAS Circular 921 Introduction to Fish Health Management. https://edis.ifas.ufl.edu/FA004.
UF/IFAS Fact Sheet FA-14 Aeromonas Infections. https://edis.ifas.ufl.edu/FA042.
UF/IFAS Fact Sheet FA-16 Ammonia. https://edis.ifas.ufl.edu/FA031.
UF/IFAS Fact Sheet FA-28 Mongenean Parasites of Fish. https://edis.ifas.ufl.edu/FA033.
UF/IFAS Fact Sheet FA-29 Introduction to Viral Diseases of Fish. https://edis.ifas.ufl.edu/FA034.
UF/IFAS Fact Sheet FA-31 Vibrio Infections of Fish. https://edis.ifas.ufl.edu/FA036.
UF/IFAS Fact Sheet FA-55 Submission of Fish for Diagnostic Evaluation. https://edis.ifas.ufl.edu/FA055.
UF/IFAS Fact Sheet FA-95 Molds in Fish Feeds and Aflatoxicosis. https://edis.ifas.ufl.edu/FA095.
UF/IFAS Fact Sheet VM-77 Use of Formalin to Control Fish Parasites. https://edis.ifas.ufl.edu/VM061.
UF/IFAS Fact Sheet VM-86 Use of Salt in Aquaculture. https://edis.ifas.ufl.edu/VM007.
UF/IFAS Fact Sheet VM-87 Sanitation Practices for Aquaculture Facilities. https://edis.ifas.ufl.edu/AE081.
UF/IFAS Fact sheet VM-90. Amyloodinium Infections in Fish. https://edis.ifas.ufl.edu/VM004.
UF/IFAS Fact Sheet VM-96 Mycobacteriosis in Fish. https://edis.ifas.ufl.edu/VM055.
UF/IFAS Fact Sheet VM-97 Fungal Diseases of Fish. https://edis.ifas.ufl.edu/VM033.
UF/IFAS Fact Sheet VM-142 Spring Viremia of Carp. https://edis.ifas.ufl.edu/VM106
References and Further Reading
Bedell G.W. 1971. "Eradicating Ceratomyxa shasta from infected water by chlorination and ultraviolet irradiation." Progressive Fish-Culturist 33: 51–54.
Collins M.T., J.B. Gratzek, D.L. Dawe and T.G. Nemetz. 1975. "Effects of parasiticides on nitrification." Journal of the Fisheries Research Board of Canada 32: 2033–2037.
Collins M.T., J.B. Gratzek, D.L. Dawe and T.G. Nemetz. 1976. "Effects of antibacterial agents on nitrification in an aquatic recirculating system." Journal of the Fisheries Research Board of Canada 33: 215–218.
Hach Company. 2002. Water Analysis Handbook, Fourth edition. Loveland, CO: Hach Company. 1260 pp.
Hoffman G.L. 1974. "Disinfection of contaminated water by ultraviolet irradiation with emphasis on whirling disease (Myxosoma cerebralis) and its effect on fish." Transactions of the American Fisheries Society 103: 541–550.
Kasai H., M. Yoshimizu and Y. Ezura. 2002. Disinfection of water for aquaculture. Fisheries Science, Volume 68, Supplement I, pp. 821–824. Oxford, UK: Blackwell Publishing.
Levine G. and T.L. Meade. 1976. The effects of disease treatment on nitrification in closed system aquaculture. Proceedings from the 7th Annual Meeting of the World Mariculture Society, J.W. Avault Jr. (editor), pp 483–493. Baton Rouge, LA: World Mariculture Society, Louisiana State University.
Miocevic I., J. Smith, L. Owens and R. Speare. 1993. "Ultraviolet sterilization of model viruses important to finfish aquaculture in Australia." Australian Veterinary Journal 70: 25–27.
Nagy R. 1964. "Application and measurement of ultraviolet irradiation." American Industrial Hygiene Association Journal 25: 274–281.
National Research Council. 1993. Nutrient Requirements of Fish (Committee on Animal Nutrition, Board on Agriculture). Washington, DC: National Academy Press. 114 pp.
Normandeau D.A. 1968. Progress Report, Project F-14-R-3, State of New Hampshire (Mimeo).
Rodriguez J. and T.R. Gregg. 1993. Considerations for the Use of Ultraviolet in Fish Culture in Techniques for Modern Aquaculture: Proceedings of an Aquaculture Engineering Conference 21–23 June 1993, p 482. American Society of Agricultural Engineers, St. Joseph, MI.
Spotte S. 1979. Fish and Invertebrate Culture: Water Management in Closed Systems, Second edition. New York, NY: John Wiley and Sons Inc. 179 pp.
Virkon Aquatic®: http://www.wchemical.com/products/biosecurity-supplies-disinfectants/virkon-auqatic/virkon-aquatic-10-lb-tub-virkdlb0010.html. Accessed August 11, 2009
Vlasenko M.I. 1969. "Ultraviolet rays as a method for the control of disease of fish eggs and young fishes." Journal of Ichthyology (formerly, Problems of Ichthyology) 9: 697–705.
Yoshimizu M., H. Takisawa and T. Kimura. 1986. "UV susceptibility of some fish pathogenic viruses." Fish Pathology 21: 47–52.