Introduction
The porkfish, Anisotremis virginicus (Figure 1), is a member of the grunt family, Haemulidae. In general, grunts are some of the most ecologically important fishes found throughout the world. As their common name implies, members of this family create a characteristic "grunting" sound by rubbing their pharyngeal teeth together, typically during periods of agitation or courtship. There are approximately 150 grunt species (over 17 genera) distributed throughout the Atlantic, Indian, and Pacific Oceans (Nelson 2006). They are common on coral and rocky reefs, ledges, and hard bottoms. The white grunt, Haemulon plumieri, has a high-value fishery and is primarily harvested by recreational anglers for its value as a food fish. In addition, many species of grunts are popular in public aquariums because they're abundantly available, and their schooling behavior and bright colors create interest in aquarium exhibits. Porkfish also have additional appeal to aquarists because they are "cleaner" fish during their juvenile phase, picking parasites from larger fish and other vertebrates (Sazima, Grossman, and Sazima 2010; Böhlke and Chapman 1993). This beneficial behavior not only cleans the other fish, but also reduces the parasite load of the entire system. Furthermore, scientists and aquarists have recently achieved a greater understanding of appropriate aquaculture protocols for grunts in general (Barden, Wittenrich, and Cassiano 2014; Ohs, DiMaggio, and Grabe 2011; Cassiano, Ohs, and Hill 2009), and specifically porkfish (Cassiano et al. 2012). These characteristics and advancements have led to porkfish being identified as a candidate species for commercial aquaculture.

Credit: George H. Burgess, Florida Museum of Natural History
Natural History
The porkfish is an abundant and important reef fish distributed throughout the Caribbean and the South American coast to Brazil, as well as Bermuda, Florida, and the east and south portions of the Gulf of Mexico (Smith 1997; Robins and Ray 1986). Porkfish have a short, deep body with a nearly straight ventral profile, a strongly arched dorsal profile, thick lips, and a blunt snout (Smith 1997). They have yellow and silver-blue horizontal stripes with two vertical black bars, one from the nape through the eye to the mouth, and the other from the origin of the dorsal fin to the base of the pectoral fin (Smith 1997). In addition, the fins are yellow (Smith 1997). Porkfish typically inhabit coral reefs, rocky bottoms, and associated habitats. They are the only grunts throughout the Caribbean that have yellow stripes with two vertical black bars, making wild-caught specimens easily identifiable within this region (Smith 1997). Porkfish typically feed at night on a variety of invertebrates, including mollusks, echinoderms, crustaceans, and worms (Smith 1997; Courtenay and Sahlman 1978). Several studies have shown that they prefer chitinous prey (47%–60% of prey consumed), to soft bodied or calcified prey (Randall 1967; Grubich 2003). Juvenile porkfish are also known to pick parasites from larger fishes and even larger aquatic animals such as sea turtles (Sazima, Grossman, and Sazima 2010; Böhlke and Chapman 1993), adding to their appeal as an aquarium fish. Juvenile porkfish, like juvenile French grunts, feed on a variety of zooplankton, including copepods, shrimp and crab larvae, amphipods and Mysis shrimp (Verweij et al. 2006). Maximum total length for porkfish is reported at 40.6 cm (16 inches) standard length, although 25 cm (10 inches) is more common (IGFA 2001; Courtenay and Sahlman 1978).
Culture Techniques
Despite their value, limited information is available on the appropriate aquaculture techniques necessary to rear porkfish. However, the University of Florida's Tropical Aquaculture Laboratory (TAL) has conducted studies on the first feeding phase of porkfish larvae and has explored preliminary nursery and grow-out protocols that will help develop commercial scale production of the species (Cassiano et al. 2012). In addition, information on the aquaculture protocols of closely related species, including pigfish and French grunts, can be used to aid in the development of appropriate aquaculture techniques for porkfish (Barden, Wittenrich, and Cassiano 2014; Ohs, DiMaggio, and Grabe 2011; Cassiano, Ohs, and Hill 2009).
Broodstock and Spawning
Porkfish, like all grunts, are pelagic spawners, which means that they disperse their eggs in the open ocean where the larvae hatch and become part of the plankton community. Porkfish rise in the water column to broadcast eggs, a behavior best characterized as a "spawning ascent." At the height of their ascent, the female releases thousands of separate, spherical eggs that the male simultaneously fertilizes. The eggs measure slightly less than 1 mm and float after fertilization. In the wild, this spawning behavior takes place at dusk with peaks during the spring and summer. Within the culture system, light cycle and temperature may be manipulated to simulate these peak spawning times.
No information could be found on when porkfish reach sexual maturity, but French grunts and pigfish are sexually mature at 12–15 cm (~5–6 inches) and 20 cm (~8 inches) total length respectively, and both by the end of their second year (Barden, Wittenrich, and Cassiano 2014; Cassiano, Ohs, and Hill 2009). Volitional spawning, without the use of hormones, is achieved by maintaining a light cycle of 12–14 hours accompanied by water temperatures of 77°F–81°F (25°C–27°C) and 32–35 g/L salinity. Although broodstock tanks of different sizes have not yet been tested, the broodstock tank likely needs to be greater than ~300 gallons to achieve volitional spawning because populations kept in smaller tanks have not spawned, even under otherwise ideal conditions. Induced spawning (oocyte manipulation using hormones) and strip spawning (manually expressing gametes and mixing in salt water to initiate fertilization) have been demonstrated with pigfish and corocoro grunts, and these methods are worth exploring in porkfish as well (Cassiano, Ohs, and Hill 2009; Mata et al. 2004; DiMaggio, Broach, and Ohs 2014). Once porkfish have acclimated to an artificial photoperiod regime, which generally takes two weeks, spawning occurs each night around the time the lights are turned off. The eggs float in seawater and are gathered by means of surface-skimming collectors that pass outgoing water through a mesh-screened collection container before traveling to the filtration sump. Eggs can also be collected by internal airlift egg collectors (Ohs et al. 2019). Nutrition is passed from adult fish to eggs and larvae, which makes developing an optimal broodstock diet that yields greater survival and growth of progeny necessary for commercial success. Broodstock diets have not yet been evaluated for porkfish, but it is recommended that a combination of pellets (containing at least 45 percent protein) be provided, along with shrimp, squid, clams, and oily marine fish flesh. To initiate spawning in pigfish, broodstock were fed a diet consisting of pellets (50 percent protein and 15 percent lipid) and frozen squid (Cassiano, Ohs, and Hill 2009).
Larviculture
Late in the evening of a spawning event or early the next morning, eggs are removed from surface collectors and placed into a ~1 L container. Within 10 to 20 minutes, the viable embryos will float to the surface, and the dead embryos, unfertilized eggs, and debris will sink to the bottom. Viable embryos (Figure 2) can then be decanted from the surface and stocked directly into larval rearing tank(s) at 15–30 embryos/liter. The water within porkfish larval tanks should be maintained between 77°F and 81°F (25°C–27°C) and 32–35 g/L salinity. An airstone placed in the bottom center of the tank will provide sufficient gentle aeration to keep embryos and newly hatched larvae in suspension (it's important to keep them in suspension as the floating eggs tend to raft together, reducing available oxygen to those in the middle). Under these conditions, hatching occurs within 24 hours after fertilization. Porkfish larvae will subsist solely on yolk sac protein and lipid reserves for the first two days after hatching. Although larvae are delicate during this stage, they undergo dramatic changes, including development and pigmentation of the eyes and the development of the mouth and digestive tract. By the end of the second day, larvae have exhausted their yolk reserves and should be provided both appropriate live prey and environmental conditions conducive to feeding (Figure 3).

Credit: Eric J. Cassiano
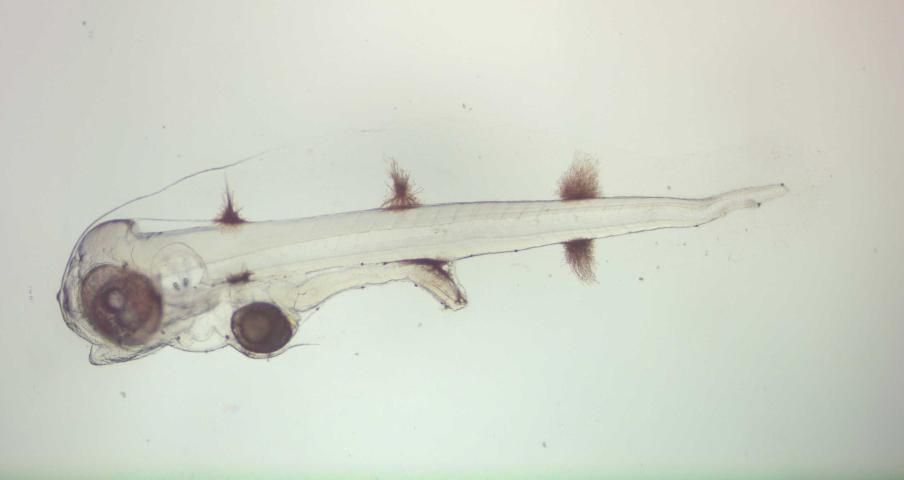
Credit: Eric J. Cassiano
Porkfish larvae have been raised in 13 L, 60 L, 350 L, and 950 L tanks at the Tropical Aquaculture Laboratory (TAL). At TAL, larval rearing tanks are connected to central filtration systems with mechanical filtration, large fluidized bed sand biological filters, protein skimmers, and UV sterilizers. External standpipes are used to adjust the working volume in each tank and draw outgoing water from the bottom of the tank. Barrel screens are placed over the outflow on the bottom of each tank to prevent larvae from entering the drain. Tanks are filled with synthetic seawater (Instant Ocean) that has been aged for 48 hours under heavy aeration.
Light intensity, light spectrum, and tank color have not been evaluated in first-feeding porkfish larvae. However, a strong feeding response is observed when using normal fluorescent lamps (6500 K double lamps suspended 10 cm [~4 inches] above the surface) and tanks with black walls and bottoms. Upon initial feeding, aeration is reduced to a gentle flow, and rotifers (Brachionus plicatilis, ~120 µm body width) are stocked at 5–10/mL. A study conducted at TAL compared the use of rotifers (B. plicatilis) and copepod nauplii as first-feeding prey items for porkfish larvae (Cassiano et al., 2012). The results of that study indicate that although growth was better for those larvae fed copepods, the increased survival of those larvae fed rotifers coupled with the ease of growing rotifers suggest that a first-feeding regime of rotifers for porkfish larvae is sufficient (Cassiano et al. 2012). Similar results have also been seen in French grunt larvae (Barden, Wittenrich, and Cassiano 2014). At this time, a shading agent such as algae paste (Nanno 3600™; Reed Mariculture) or live microalgae (Isochrysis galbana or Nannochloropsis oculata) is also added to each tank to a density of 100,000–300,000 cells/mL to provide a background contrast that aids in feeding success of larvae. The use of live microalgae is reported to also help combat increases in nitrogenous waste, a problematic by-product of metabolism. A gentle flow of water from the recirculating filtration system can be initiated as early as 2 days post hatch (dph), or it can be postponed until 4 or 5 dph, depending on the need to reduce nitrogenous waste buildup (especially ammonia). Aeration is slightly increased on 4–5 dph. Every day the live rotifer density should be checked and, if needed, supplemented with new prey if densities fall below 5/ml. This becomes more apparent as the larvae grow and the water flow increases.
On approximately 10 dph, newly hatched Artemia nauplii are added at a density of 0.5–1/mL. Also at this time, aeration and water exchange are increased in order to maintain high oxygen levels and reduce waste build-up in the tank. The feeding of rotifers and the inclusion of a shading agent can be discontinued by ~15 dph. Pellets can begin to be introduced around 20 dph each morning, before or with Artemia nauplii to facilitate weaning. Begin with a smaller pellet (Otohime B1; 250–360 µm), and move to a larger one over a few days (Otohime B2; 360–650 µm) or use an equal mixture of both during weaning. This diet is a dry feed that contains 65 percent protein and 10 percent fat. All fish can be weaned onto a pellet diet by approximately 30 dph, at which time the feeding of Artemia is discontinued. Under these conditions, metamorphosis occurs between 25–30 dph when individuals undergo morphological and behavioral changes that result in juvenile fish (Figure 4).

Credit: Matthew L. Wittenrich
Growout
Juveniles (~45 dph) can be transferred to grow-out tanks a few weeks after having been weaned onto a pellet diet. Since early juveniles have high metabolic demands, pelleted feeds of approximately 50 percent protein should be offered several times throughout the day or offered by means of an automatic feeder. Growth is rapid, with juveniles reaching 5 cm (~2 inches) by the end of the first month and 6–7 cm (~3 inches) by the end of three months.
Market
The market value of porkfish has not been fully evaluated. They are primarily harvested recreationally for display in public and private aquariums. One aquaculture producer has an arrangement with a public display aquarium wherein collected eggs, and subsequently hatched larvae, are grown to the juvenile phase (~2 inches) at their production facility and returned to the home aquarium for a fee. There is particular interest from large public display aquarists for both the adult and juvenile phases. The juveniles are valued for their coloration and for the cleaner services they provide larger fish and other vertebrates (Figure 4).
Conclusion
Porkfish are well suited to current commercial aquaculture techniques. The larvae are relatively large at first feeding, and they readily accept rotifers at first feeding and Artemia nauplii as a secondary diet. Larvae grow quickly and begin accepting a pellet diet within the first three weeks of hatching. Juvenile growth continues to be rapid, with fish attaining 6–7 cm (~3 inches) in just under 3 months. Porkfish have a unique striking coloration which appeals to aquarists; this coupled with their ease of culture make them a viable candidate for marine ornamental aquaculture.
Acknowledgements
Special thanks to Rising Tide Conservation and the Association of Zoos and Aquariums for funding this work. Special thanks also to Gary Violetta, Justin Zimmerman, and Jim Kinsler of SeaWorld Orlando as well as the staff and faculty at UF's Tropical Aquaculture Laboratory.
References and Recommended Readings
Barden, K. P., M. L. Wittenrich, and E. J. Cassiano. 2014. Candidate species for marine ornamental aquaculture: French grunt, Haemulon flavolineatum. FA186. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/fa186
Böhlke, J. E., and C. C. G. Chaplin. 1993. Fishes of the Bahamas and adjacent tropical waters. 2nd edition. University of Texas Press, Austin, TX.
Cassiano, E. J., C. L. Ohs, and J. E. Hill. 2009. Candidate species for Florida aquaculture: Pigfish, Orthopristis chrysoptera. FA160. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/fa160
Cassiano, E. J., M. L. Wittenrich, G. C. Violetta, and C. A. Watson. 2012. "Growth and survival of porkfish (Anisotremus virginicus) larvae: comparing rotifers and copepod nauplii during first feeding." Animal Biology & Animal Husbandry 4(2): 72–78.
Courtenay, W. R., and H. F. Sahlman. 1978. Pomadasyidae. In: Fisher, W. (ed.) FAO species identification sheets for fishery purposes. Western Central Atlantic (Fishing Area 31), Volume 4. FAO, Rome.
DiMaggio, M. A., J. S. Broach, and C. L. Ohs. 2014. "Evaluation of ovaprim and human chorionic gonadotropin doses on spawning induction and egg and larval quality of pigfish, Orthopristis chrysoptera." Journal of World Aquaculture Society, 45:3, 243-257.
DiMaggio, M. A., J. S. Broach, C. L. Ohs, and S. W. Grabe. 2013. "Captive volitional spawning and larval rearing of pigfish." North American Journal of Aquaculture, 75:1, 109–113.
Grubich, J. 2003. "Morphological convergence of pharyngeal jaw structure in durophagous perciform fish." Biological Journal of the Linnean Society, 80:147–165.
IGFA. 2001. Database of IGFA angling records until 2001. IGFA, Fort Lauderdale, Florida.
Mata, E., J. Rosa,. A. Velasquez, and T. Cabrera. 2004. "Hormone to induce spawning and larval description of corocoro, Orthopristis ruber Cuvier (Pisces: Haemulidae)." Journal of Marine Biology and Oceanography 39(1):21–29.
Nelson, J. 2006. Fishes of the world (4th Ed.). John Wiley & Sons, Inc., Hoboken, New Jersey, 601 pp.
Ohs, C. L., M. A. DiMaggio, and S. W. Grabe. 2011. Species profile: Pigfish, Orthopristis chrysoptera. Southern Regional Aquaculture Center publication no. 7209. Texas A&M University, TX. https://srac.tamu.edu/categories/view/33
Ohs, C.L., J.S. Broach, E.J. Cassiano, and J. Marcellus. 2019 Design and Operation of Egg Collectors for Pelagic Spawning Marine Fishes. FA211. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/fa211
Randall, J. E. 1967. "Food habits of reef fishes of the West Indies." Studies in Tropical Oceanography 5: 665–847.
Robins, C. R., and G. C. Ray. 1986. A field guide to Atlantic coast fishes of North America. Houghton Mifflin Company, Boston, Massachusetts. 354 p.
Sazima, C., A. Grossman, and I. Sazima. 2010. "Turtle cleaners: reef fishes foraging on epibionts of sea turtles in the tropical Southwestern Atlantic, with a summary of this association type." Neotropical Ichthyology 8: 187-192.
Smith, C. L. 1997. National audubon society field guide to tropical marine fishes (4th Ed.). Random House, Inc. New York, New York. 718 pp.
Verweij, M. C., I. Nagelkerken, S. L. Wartenbergh, I. R. Pen, and G. van der Velde. 2006. "Caribbean mangroves and seagrass beds as daytime feeding habitats for juvenile French grunts, Haemulon flavolineatum." Marine Biology 149(6): 1291–1299.