Introduction
Most soils used for commercial crop production in Florida are sandy by nature and are classified as sands, fine sands, or sandy loams. These sandy soils are often water-repellent or hard-to-wet, which can pose a major challenge to growers. The hard-to-wet or water-repellent nature of such soils arises from the fact that they are often hydrophobic (derived from the ancient Greek hydro: water and phobos: fear) or difficult to wet once they are dry.
Water-repellent soils are unable to effectively adsorb or retain water. On these soils, water may simply pool on the surface or may move down preferred pathways, leaving large amounts of soil dry even when a large volume of water is applied. Thus, managing water and nutrients on sandy soils is often challenging.
According to the scientific literature, water-repellent soils have been found and studied in 21 states in the United States. Florida is one of the states with the earliest reports of water-repellent sandy soils (Jamison 1942; Dekker, Oostindie, and Ritsema 2005; Oostindie et al. 2012). In the 1940s, Science published a paper titled An Interpretation of the Cause of Water-Repellent Sandy Soils Found in Citrus Groves of Central Florida (Wander 1949). Since that time, researchers have studied ways to help growers deal with the challenges of water-repellent sandy soils, and recently UF/IFAS scientists reported how to alleviate soil water repellency using surfactants (Park et al. 2004). This article provides an overview of surfactants and how they may be used to better manage water and nutrients in sandy soils for vegetable and fruit production.
What problems are caused by water-repellent sandy soils?
Water repellency causes reduced and uneven infiltration of water into soils and results in poor crop yield. Blackwell et al. estimated that water repellency caused an annual economic loss in crop production by 40% (quoted in Ghadim 2003). The following problems are caused by water repellency in sandy soil and result in economic losses (Doerr and Thomas 2000; Wahl 2008; Hall 2009).
- Rapid leaching of surface-applied agrichemicals
- Loss of water and nutrient availability
- Uneven distribution of nutrients and water
- High soil evaporation
- Severe runoff
- Soil erosion
- Low productivity
Why are sandy soils water-repellent?
Water repellency is determined by the properties of the outer surface of the organic coatings on soil particles. Amphipathic or amphiphilic (derived from the Greek amphis: both and philia: love, friendship) compounds are key constituents of the organic component of the outer layer of soil particles. These compounds have both polar and nonpolar components—they attract water at one end and repel it at the other. A few possible mechanisms are usually considered to be responsible for water repellency in sandy soils (Horne and McIntosh 2003; Hallett 2008):
- Changes in molecular orientation of organic compounds. The amphipathic compounds change orientation when sandy soils become dry. In the wetted state, these compounds usually have their polar (water-attracting) ends pointing outwards. However, when soils become dehydrated, there is a reconfiguration or reorientation of the compounds so that these amphipathic compounds may present a hydrophobic end on the surface.
- Changes in ionization of functional groups in organic compounds. Under moist conditions, the functional groups are ionized and hydrophilic (derived from the Attic Greek hydro: water and philia: love). However, when soil is dry, the functional groups become protonated and hydrophobic.
- Changes in the screening of hydrophobic compounds. Under moist conditions, the hydrophobic material is effectively screened or covered, but water repellency develops when the soil dries and the hydrophobic compounds become more exposed (Figure 1).
Water repellency in soils can be alleviated by applying a surfactant.
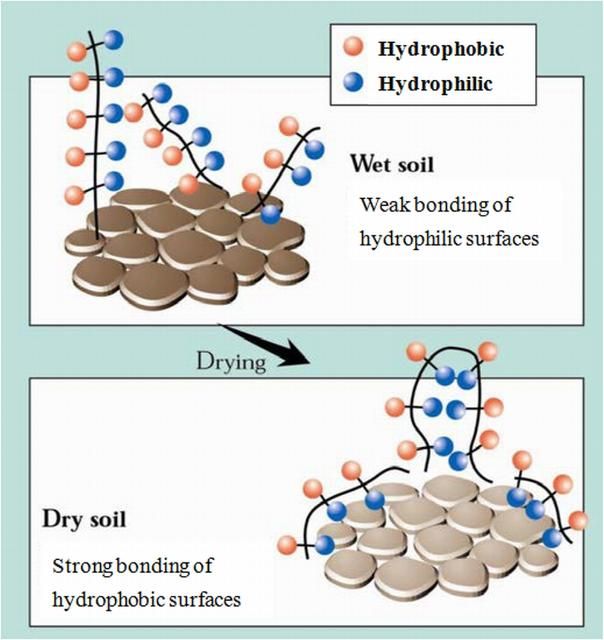
Credit: Hallett (2008)
How can we characterize soil water repellency?
There are a few methods to measure soil water repellency. The most commonly used method is the water drop penetration time. In this method, if a water drop does not enter the soil spontaneously, the soil is water-repellent because its soil-water contact angle is >90°. The procedure for this method is to place a drop of water on the soil and observe. If the water penetrates the soil impulsively, the soil is NOT water-repellent; otherwise, it is water-repellent. This method is almost always used because of its simplicity (Letey, Carrillo, and Pang 2003; Madsen, Coronel, and Hopkins 2012).
What is a surfactant?
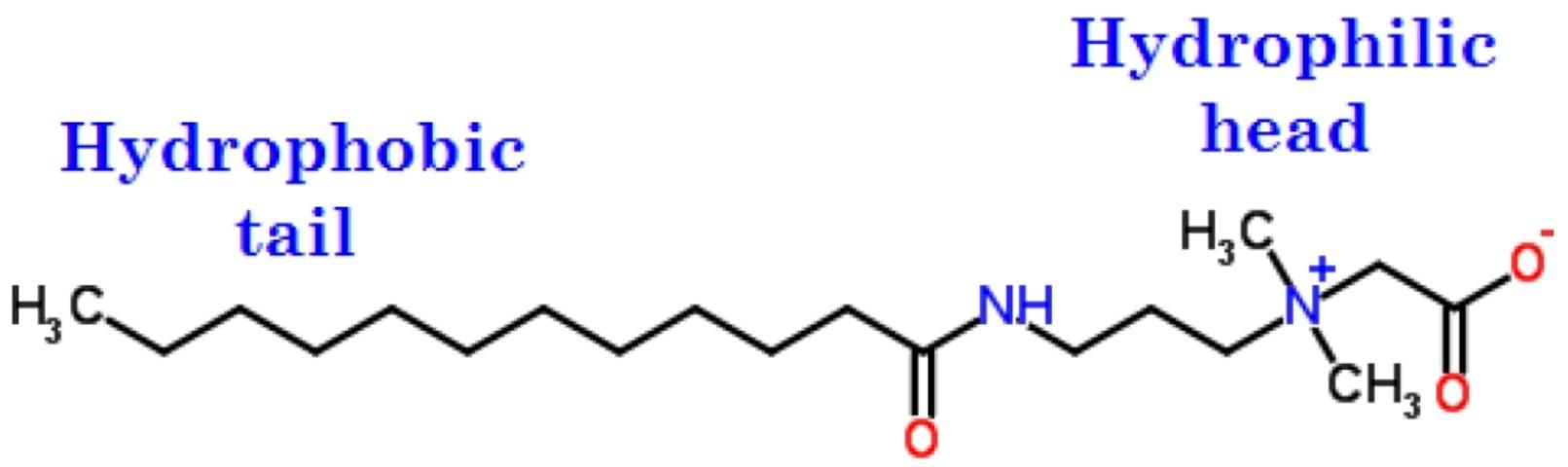
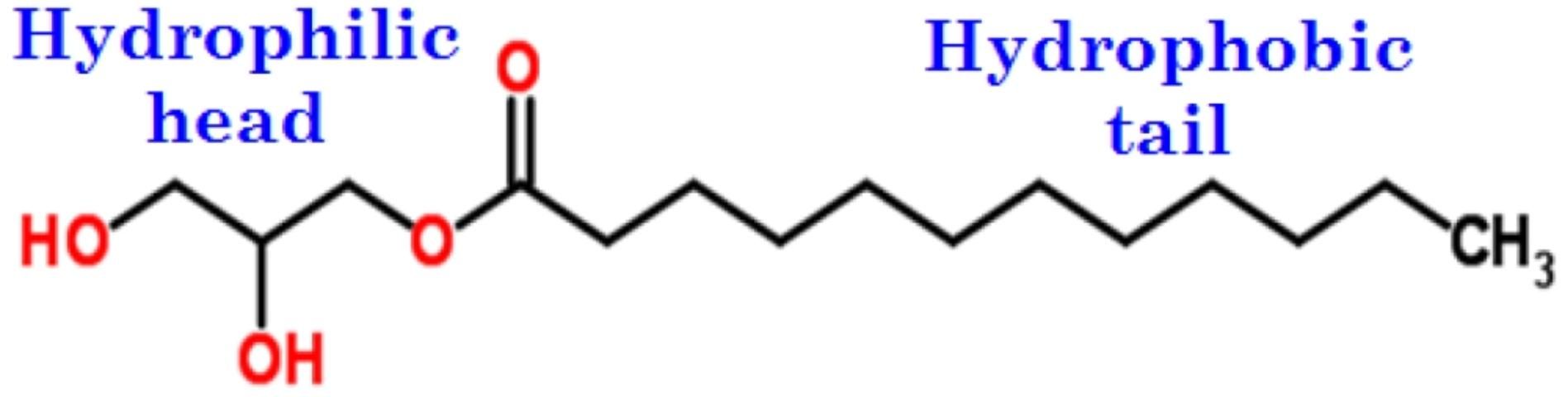
A surfactant is a surface-active agent, also known as a wetting agent, which, when used in a small quantity, distinctly affects the surface characteristics of a system. Soaps and chemical detergents are typical examples of surfactants because of their dual (also known as amphipathic) character. A surfactant consists of a hydrophilic polar head group, often ionic, and a hydrophobic tail, usually a long-chain hydrocarbon (Figure 2). A surfactant has an affinity for either oils (hydrophobic) or water (hydrophilic) and acts as a wetting agent to introduce a degree of continuity between water and soil particles. A surfactant can be used to reduce the surface tension of a liquid (such as water), the interfacial tension between two liquids, or the tension between a liquid and solid (such as water and soil). This property allows a surfactant to be mixed or dispersed readily in water or other liquids. Surfactants reduce the surface tension of water, allowing it to penetrate and wet soils more easily and evenly. Thus, a surfactant can promote the absorption and retention of moisture in soil.
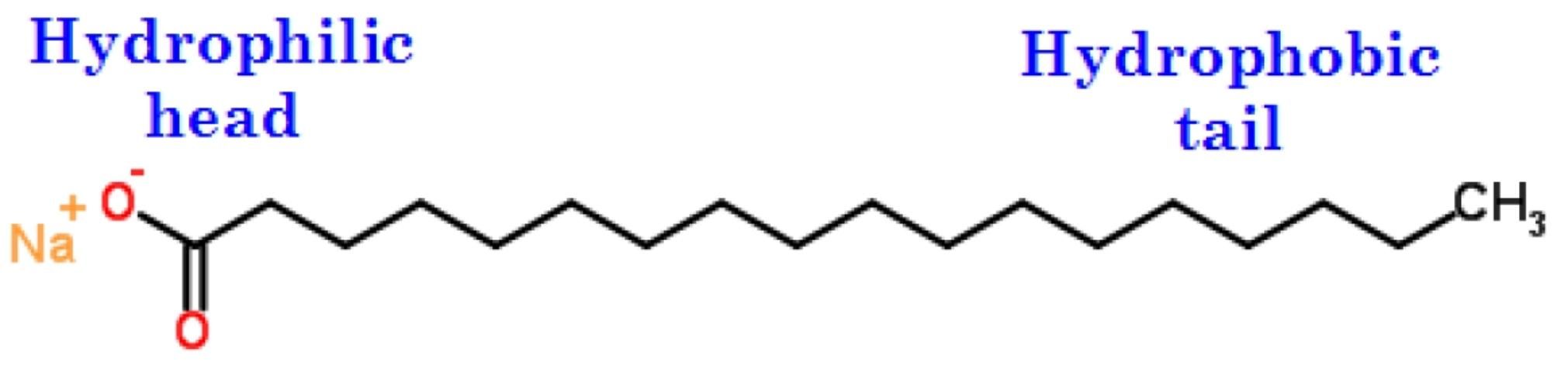
Credit: Adapted from http://www.chemspider.com
How many types of surfactants are available?
There are many natural and artificial surfactants. Most of them have chemically similar hydrophobic tails consisting of a long hydrocarbon chain as Figure 2 shows, which can be linear, branched, or aromatic. Some may have fluorocarbon chains (e.g., organofluorine compounds) or siloxane chains (e.g., organosilicon compounds). However, their hydrophilic heads are distinctly different from each other. Some are charged negatively, some positively, some amphoterically (derived from the Greek, amphoteroi: both), and some are not charged. Based on the electrical properties of the heads, surfactants can be classified into four different types (Figure 3): anionic, cationic, amphoteric, and nonionic.
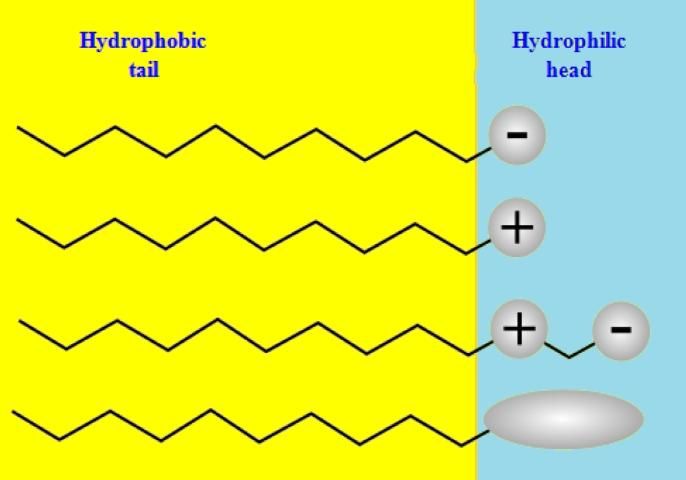
Credit: Adapted from https://en.wikipedia.org/wiki/Surfactant
1. Anionic surfactants
Surfactants of this type contain anionic functional groups at their heads. The soap in Figure 2 belongs to this type. The sodium searate in soap can dissociate, and the sodium forms free positive ions when it contacts water. Soap is a carboxylatic surfactant (Figure 2). Other anionic heads include sulfates such as sodium dodecyl sulfate, a common component of many cleaning products. Since anionic surfactants are negatively charged, they increase the retention of cations.
2. Cationic surfactants
The charge of this type of surfactant is pH-dependent because the cationic functional group is dependent on the dissociation of amines (Figure 4). Surfactants with primary, secondary, or tertiary amines are all positively charged when pH is less than 10.0, while those with a quaternary ammonium cation are always positively charged, regardless of pH. A typical example of this type of surfactant related to agriculture is cetylpyridinium chloride (CPC with the molecular formula of C21H38NCl), which is a cationic quaternary ammonium compound used as an ingredient in certain pesticides. Cationic surfactants favor the retention of anionic nutrients such as nitrate-nitrogen and phosphorus.
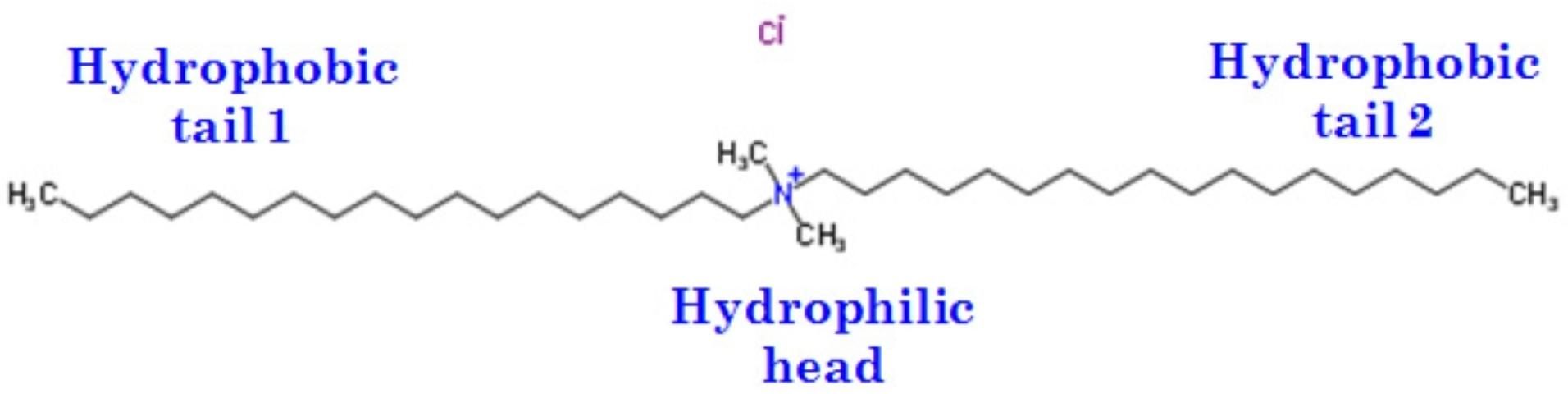
Credit: Adapted from http://www.chemspider.com
3. Amphoteric surfactants
This type of surfactant has the characteristics of both an acid and a base, and is capable of reacting as either an anionic or cationic surfactant (Figure 5). They are also known as zwitterionic surfactants (derived from German, zwitter: hybrid) and were formerly called dipolar ions because they have both cationic and anionic centers attached to the same molecule and carry both a positive and a negative charge. The cationic part is based on primary, secondary, or tertiary amines or quaternary ammonium cations, while the anionic part can be more variable and include sulfonates, as in CHAPS (3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate).
Credit: Adapted from http://www.chemspider.com.
4. Nonionic surfactants
This type of surfactant is an adjuvant with no electrical charge (Figure 6). These surfactants are widely used in agriculture because they are compatible with all types of pesticides. They usually have a long alcohol chain with a hydrophilic alcohol head and hydrocarbon tail that is hydrophobic (i.e., lipophilic). Among them, the fatty alcohols, such as cetyl alcohol, are prominent.
Humectants such as glycerol can also promote retention of moisture and because they are hygroscopic, can be added to another substance or material to keep it moist. Hygroscopic compounds have high affinity for water and are often used as desiccants. However, they are not surfactants.
In theory, all four types of surfactants can be used for improving nutrient- and water-use efficiencies. However, their costs differ significantly due to the differences in chemical structure and composition. For improving nutrient- and water-use efficiencies and minimizing offsite impacts of fertilization, the most economic surfactant should be selected and used. Additionally, there are novel surfactants, but they are not included in this article.
Credit: Adapted from http://www.chemspider.com Accessed on February 5, 2024.
How does a surfactant work in sandy soils?
Water repellency is caused by the structure and composition of soil, as well as other factors. Coarse soils with low clay content are more prone to water repellency. Soil particles in sandy soils are greater than 50 µm in size and have low surface area. Thus, their adsorptive area is low. In addition, their low surface area makes it highly possible for hydrophobic waxes from plants and soil microbes to coat the coarse soil particles. These coatings significantly contribute to soil water repellency. Quartz is by far the most common component in sandy soils and is a crystalline form of silicon dioxide (SiO2), also known as silica. As a soil particle, silica itself is not water-repellent, but it can self-organize and become water-repellent by reacting with organic waxes from plants and microbes when the soil becomes dry (McHale et al. 2007). Other factors such as low organic matter levels also contribute to sandy soil's water repellency. Fire can burn off soil organic matter and induce soil water repellency (DeBano 2000). Water repellency affects the wetting pattern of soil and may result in an uneven wetting pattern. It can also result in large yield reductions in dry seasons and contribute to late starts to the growing season.
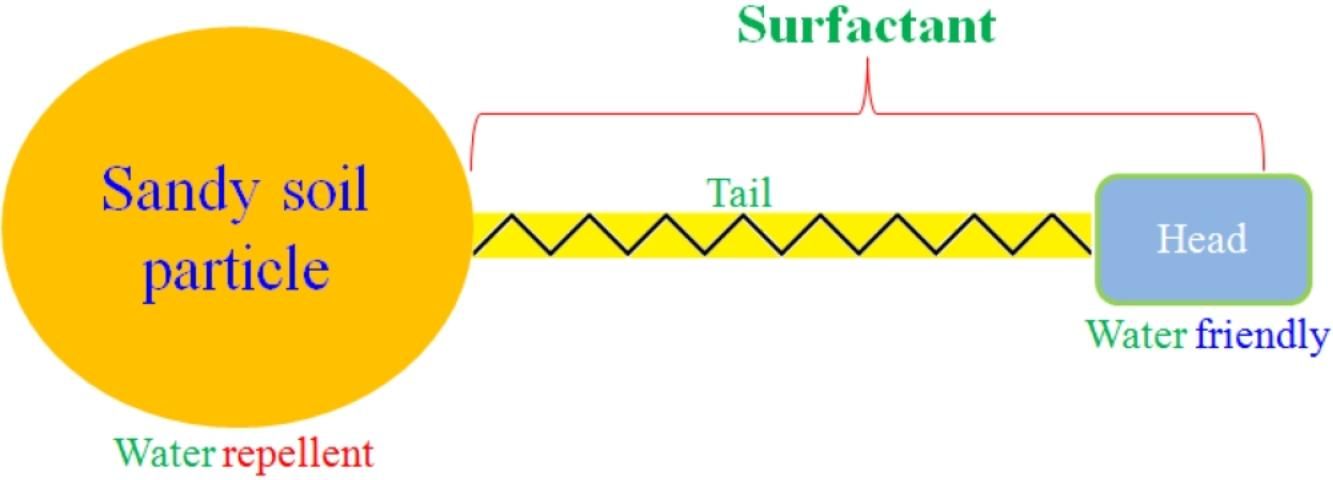
A surfactant's structural characteristics determine its compatibility to both hydrophilic and lipophilic materials in soil. Thus, surfactants can react with both water-friendly and water-repellent soil particles (Figure 7). Therefore, surfactants can significantly improve sandy soil by enhancing the soil's water retention and nutrient-holding capacity (Ghebru, duToit, and Steyn 2007). For example, a surfactant increases potato petiole nitrate-nitrogen (NO3-N) by 28.9% from 4.5 to 5.8 g/kg 75 days after emergence (Arriaga, Lowery, and Kelling 2009). With surfactants, commercially grown vegetable crops can obtain water and nutrients more efficiently (Sarvaš 2003). These effects of surfactants on soil quality may enhance yield and profitability for commercial crop production in Florida's sandy soils.
Credit: Guodong Liu, UF/IFAS

Credit: Guodong Liu, UF/IFAS
What surfactants should you choose for crop production?
There are many surfactants available on the market today (Madsen, Coronel, and Hopkins 2012). You need to take a few factors into account to determine the appropriate surfactant to use in crop production. The surfactant you choose needs to be effective to minimize soil water repellency. Some surfactants may have a double effect, both reducing water surface tension and increasing water retention. For example, one surfactant recently used in Florida, Stockosorb, can reduce water surface tension and also forms a hydrogen gel to hold water and nutrients in the root zone. These kinds of surfactants may have a better effect than those that cannot form a hydrogen gel. The surfactant you choose must be also cost-effective. The best way to find out the cost-effectiveness is to compare different surfactants and their descriptions from the market and pick the most economic and effective one for your crop production. For more information, please consult your county UF/IFAS Extension agent (see http://sfyl.ifas.ufl.edu/find-your-local-office/ accessed on February 5, 2024.).
Summary
- Water repellency can be a major problem on sandy soil, causing rapid runoff, nutrient leaching, and yield loss. Water and nutrient management on sandy soil can be a challenge to growers.
- Dehydration (drying) causes changes in orientation of amphipathic compounds on sandy soil particles and frequently results in water repellency occurring in dry soils.
- Surfactants are amphipathic in nature with both hydrophobic and hydrophilic characteristics. Soil water repellency can be alleviated with surfactants, which have a hydrophilic head and a hydrophobic tail.
- There are four types of surfactants based on the property of their polar heads: nonionic, cationic, anionic, and amphoteric (i.e., zwitterionic). All of them have the potential to be used in nutrient and water management for crop production.
- A small amount of surfactant can significantly improve soil wetting characteristics and increase the capacity of water retention and nutrient holding in sandy soils.
Acknowledgements
Dr. Paul Hallett at Scottish Crop Research Institute, UK, kindly provided Figure 1. Evonic Industries provided Stockosorb 660. Dr. Lincoln Zotarelli, UF/IFAS, provided constructive suggestions and comments to improve the manuscript.
References
Arriaga, F. J., B. Lowery, and K. A. Kelling. 2009. "Surfactant Impact on Nitrogen Utilization and Leaching in Potatoes." American Journal of Potato Research 86 (5): 383–390. https://link.springer.com/article/10.1007%2Fs12230-009-9093-z. Accessed on February 5, 2024.
DeBano, L. F. 2000. "Water Repellency in Soils: A Historical Overview." Journal of Hydrology 231-232: 4–32. https://www.sciencedirect.com/science/article/pii/S0022169400001803?via%3Dihub. Accessed on February 5, 2024.
Dekker, L. W., K. Oostindie, and C. J. Ritsema. 2005. "Exponential Increase of Publications Related to Soil Water Repellency." Australian Journal of Soil Research 43: 403–441. https://www.publish.csiro.au/sr/pdf/SR05007. Accessed on February 5, 2024.
Doerr, S. H., and A. D. Thomas. 2000. "The Role of Soil Moisture in Controlling Water Repellency: New Evidence from Forest Soils in Portugal." Journal of Hydrology 231-232: 134–147. https://www.sciencedirect.com/science/article/pii/S0022169400001906?via%3Dihub. Accessed on February 5, 2024.
Ghadim, A. K. A. 2003. "Water Repellency: A Whole-Farm Bio-Economic Perspective." In Soil Water Repellency: Occurrence, Consequences, and Amelioration, edited by C. J. Ritsema and L. W. Dekker, 303–312. Amsterdam, The Netherlands: Elsevier Science. https://www.sciencedirect.com/science/article/pii/B9780444512697500308?via%3Dihub. Accessed on February 5, 2024.
Ghebru, M. G., E. S. duToit, and J. M. Steyn. 2007. "Water and Nutrient Retention by Aquasoil® and Stockosorb® Polymers." South African Journal of Plant and Soil 24 (1): 32–36. https://www.tandfonline.com/doi/abs/10.1080/02571862.2007.10634778. Accessed on February 5, 2024.
Hall, D. 2009. "Water Repellence." Managing South Coast Sandplain Soils to Yield Potential. Department of Agriculture and Food, Western Australia (DAFWA), Bulletin 4773: 48–63.
Hallett, P. D. 2008. "A Brief Overview of the Causes, Impacts and Amelioration of Soil Water Repellency: A Review." Soil and Water Research 3 (Special Issue 1): S21–S29.
Horne, D. J., and J. C. McIntosh. 2003. "Hydrophobic Compounds in Sands from New Zealand." In Soil Water Repellency: Occurrence, Consequences, and Amelioration, edited by C. J. Ritsema and L. W. Dekker, 25–36. Amsterdam, The Netherlands: Elsevier Science. https://www.sciencedirect.com/science/article/pii/B9780444512697500059?via%3Dihub. Accessed on February 5, 2024.
Jamison, V. C. 1942. "The Slow Reversible Drying of Sandy Surface Soils Beneath Citrus Trees in Central Florida." Soil Science Society of America Proceedings 7: 36–41.
Letey, J., M. J. K. Carrillo, and X. P. Pang. 2003. "Characterizing the Degree of Repellency." In Soil Water Repellency: Occurrence, Consequences, and Amelioration, edited by C. J. Ritsema and L. W. Dekker, 51–56. Amsterdam, The Netherlands: Elsevier Science. https://www.sciencedirect.com/science/article/pii/B9780444512697500072?via%3Dihub. Accessed on February 5, 2024.
Madsen, M. D., E. G. Coronel, and B. G. Hopkins. 2012. "Soil Surfactant Products for Improving Hydrologic Function in Post-Fire Water-Repellent Soil." Soil Science Society of America Journal 77 (5): 1825–1830.
McHale, G., N. J. Shirtcliffe, M. I. Newton, and F. B. Pyatt. 2007. "Self-Organization of Hydrophobic Soil and Granular Surfaces." Applied Physics Letters 90 (5): 054110–054110-3. https://pubs.aip.org/aip/apl/article-abstract/90/5/054110/333923/Self-organization-of-hydrophobic-soil-and-granular?redirectedFrom=fulltext. Accessed on February 5, 2024.
Oostindie, K., L. W. Dekker, J. G. Wesseling, C. J. Ritsema, and D. Moore. 2012. Influence of a Single Soil Surfactant Application on Potato Ridge Moisture Dynamics and Crop Yield in a Water Repellent Sandy Soil. I. (ISHS) 938: 341–346. http://www.actahort.org/books/938/938_44.htm. Accessed on February 5, 2024.
Park, D. M., J. L. Cisar, K. E. Williams, and G. H. Snyder. 2004. "Alleviation of Soil Water Repellency in Sand Based Bermudagrass in South Florida." Acta Hortculturae 661: 111–115. https://www.actahort.org/books/661/661_13.htm. Accessed on February 5, 2024.
Sarvaš, M. 2003. "Effect of Desiccation on the Root System of Norway Spruce (Picea abies [L.] Karst.) Seedlings and a Possibility of Using Hydrogel STOCKOSORB® for Its Protection." Journal of Forest Science 49 (11): 531–536.
Wahl, N. A. 2008. "Variability of Water Repellency in Sandy Forest Soils under Broadleaves and Conifers in North-Western Jutland/Denmark." Soil & Water Research (Special Issue 1): S155–S164.
Wander, I. W. 1949. "An Interpretation of the Cause of Water-Repellent Sandy Soils Found in Citrus Groves of Central Florida." Science 110 (2856): 299–300. https://www.jstor.org/stable/1676144#metadata_info_tab_contents. Accessed on February 5, 2024.