Soil pH is one of the most important soil chemical properties and affects nutrient bioavailability and microbial activity (Liu and Hanlon 2018). The crop response to soil pH varies due to crop genetic diversity (Kering and Kaps 2004; Havlin et al. 2005; Splittstoesser 1990). Soil pH determines nutrient bioavailability and hence fruit growth, yield, and quality. The purpose of this article is to provide an overview for faculty, crop consultants, crop advisors, fruit growers, and students who are interested in fruit production.
The productive soil pH range for most fruit crops is quite wide and is affected by the composition of the soil: pH 5.4 to 6.2 for organic soils; pH 5.8 to 7.0 for mineral soils (Retamales and Hancock 2012). However, blueberries prefer acidic soil conditions and grow best between pH 4.5 and 5.5. Highbush blueberry had significantly more flowers and hence greater yields at pH 4.3 than at pH 3.4 or at pH 6.0 (Herath 1967). To maximize the profitability of fruit crop production in an environmentally sustainable manner, optimum soil pH is essential. For example, nutrient bioavailability for citrus is optimal in mineral soils with a pH of approximately 6.0. The pH of Florida soils used for commercial citrus production is typically between 5.0 and 8.4. When soil pH is lower than 5.5, the application of either calcitic lime (calcium carbonate) or dolomitic lime (calcium plus magnesium carbonate) is usually needed for optimum production (Tucker et al. 2007). Similarly, muscadine grapes perform best in a soil pH of 6.0 (Childers et al. 1995).
Because soil pH is important for commercial fruit production, orchards or groves with either young trees or bearing trees should test soil pH once every one to two years. Proper soil sampling is extremely important for getting representative results of the tested orchards. Soil sampling procedures are available in EDIS publication SL190, Soil Sampling Strategies for Precision Agriculture (Mylavarapu and Lee 2017). To diagnose nutrient bioavailability, please refer to EDIS publication, Diagnostic Nutrient Testing for Commercial Citrus in Florida (Mylavarapu et al. 2022).
Effects of Soil pH on Nutrient Bioavailability
The solubility of aluminum, iron, or manganese can be very high and may reach toxic levels at pH 5.5 or lower. These high levels of aluminum and iron ions may tie up with phosphate and minimize phosphorus bioavailability. Meanwhile, calcium or magnesium may not be adequate to meet crops' requirements. Similarly, when pH is greater than 7.5, the bioavailability of calcium or magnesium can be more than sufficient, but that of iron or manganese may not be satisfactory to feed crops. Similarly, active calcium and magnesium ions can precipitate phosphate out and may cause phosphorus deficiency of crops grown on the high-pH soil.
Soil pH determines the chemical form and solubility of nutrients. For example, iron has two different ionic forms in soil solution: ferric ion (Fe3+) and ferrous ion (Fe2+). Only the ferrous form is bioavailable for most fruit crops. However, Fe2+ may readily be oxidized to Fe3+ when soil pH is greater than 5.3. To protect the ferrous iron from oxidization, chelated iron is usually used for crop production (see EDIS publication HS1208, Understanding and Applying Chelated Fertilizers Effectively Based on Soil pH [Liu et al. 2022]. If the soil is low in iron, then iron fertilization is needed.
Boron bioavailability to fruit crops decreases with an increase in pH, particularly in calcareous soils and soils with appreciable clay content. This decrease of boron bioavailability may be attributed to the formation of B(OH)4- and anion adsorption (Marschner 1995). Another example for nutrient bioavailability related to soil pH is phosphorus (P). When soil pH is lower than 5.5 or greater than 7.5, iron and aluminum or calcium and magnesium can tie up phosphate ions, and phosphorus becomes unavailable to crops as previously mentioned. For more information on this topic, please see EDIS publication HS1207, Soil pH Range for Optimum Commercial Vegetable Production (Liu and Hanlon 2022).
Soil pH also influences microbial activities that are closely related to nutrient bioavailability. Among microorganisms, many beneficial bacteria like neutral to weakly alkaline conditions. Many fungi prefer neutral and weakly acidic conditions (Larcher 2003). If soil pH is too acidic or too alkaline, the beneficial bacteria or fungi cannot benefit fruit crops. Fruit crops grown in alkaline soils suffer from iron deficiency. Plant-growth-promoting rhizobacteria, such as Pseudomonas fluorescens, growing in low-iron conditions produce siderophores. Siderophores, (Creek: "ion carriers"), are high-affinity iron-chelating compounds that facilitate transporting iron into crop cells and stimulate plant growth (Burr et al. 1984).
Soil pH Range in the Commercial Fruit Production Areas of the State
Florida soils are diverse. The soil pH range in Florida's orchards can be as wide as 4.8 units (i.e., from pH 3.6 to pH 8.4) (USDA 1985, 1989, 1990a, and 1990b). Alachua County is an important blueberry production area, and its soil pH ranges from 3.6 to 8.4 (Table 1). Polk, Hendry, and Highlands are the top three counties in fruit production in Florida, particularly in citrus production. The soil pH in these three counties also ranges from 3.6 to 8.4 (Tables 2 through 4).
Due to genetic diversity, fruit crop species differ in their optimum pH for plant growth and development. For example, research has shown that newly planted blueberry does best in acidic soil pH ranging from 4.5 to 5.5 for high bush blueberry and 4.2 to 5.0 for rabbiteye blueberry (Retamales and Hancock 2012). However, peach has been shown to be productive between pH levels of 6.0 to 7.5 (Figure 1).
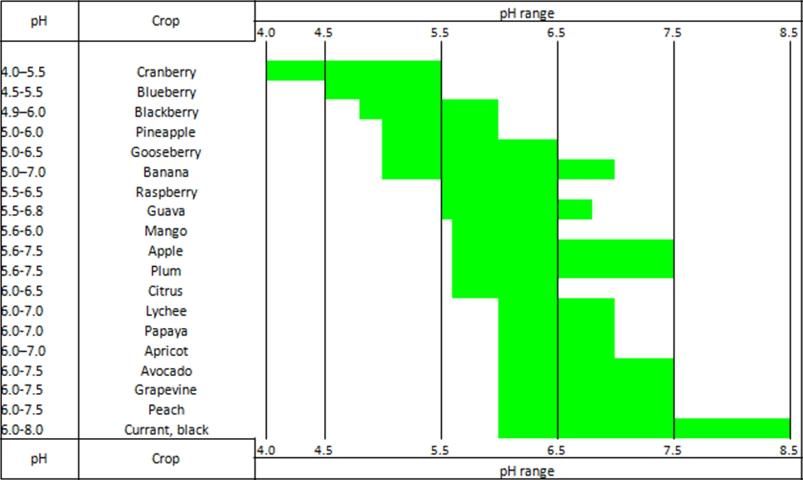
Credit: Havlin et al. (2005); Obreza and Collins (2002); Splittstoesser (1990)
Soil pH Management
Because most fruit crops grow well in a soil pH range from 5.5 to 7.0, neither too high nor too low soil pH is good for them (Figure 1). However, in reality, soil pH is seldom within the optimum range. If plants have not been established, then alternatives should be considered: using alternative land with a suitable soil pH, selecting a fruit crop that can thrive in the existing soil pH, or creating a soil pH map of the land to decide if the variability of the soil pH will allow intercropping to use soils not immediately amenable to fruit crops. Using land with soil pH outside the optimum range for the selected fruit crop requires a higher level of management, including adjusting the soil pH. There are two ways to adjust unfavorable soil pH to the proper pH range.
Nutrient Management
To adjust soil pH to a favorable range, soils are amended with agricultural lime and sulfur or dilute acids (Tagliavini et al. 2008). The lime application increases soil pH, and the sulfur or acid application reduces soil pH (Havlin et al. 2005). These two methods change the whole plow layer's pH (Figure 2) for relatively short periods of time, usually 1–3 years in most of Florida. Periodic soil testing is recommended for amended soils. Soil test results provide the current soil pH information and indicate whether there is a need for further addition of lime, sulfur or dilute acids. Acid neutralization takes time, so lime should be applied to acidic soils 3–6 months before planting or seeding. Over the course of the growing season, the pH of the limed soil may change back; therefore, periodic soil testing is recommended.
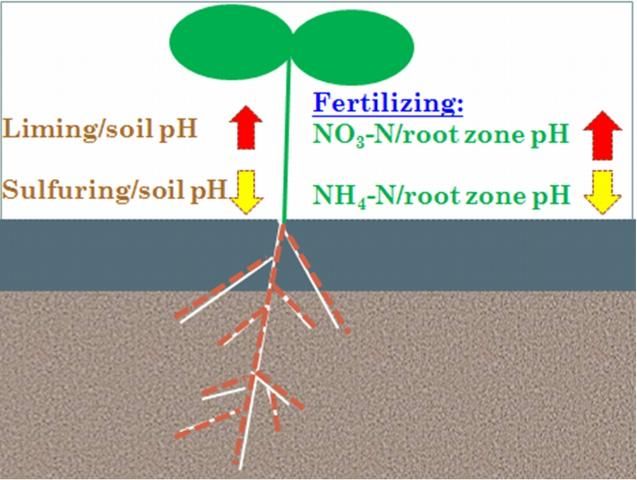
Credit: Wei Chieh Lee and Guodong Liu, UF/IFAS
Lowering the pH of alkaline soils is usually much more difficult. Elemental sulfur or acid additions may be used to lower soil pH temporarily (Shober 2011). Special attention must be paid if elemental sulfur is applied in orchards or groves. After application, elemental sulfur is oxidized into sulfuric acid and neutralizes soil alkalinity. In effect, the sulfuric acid dissolves the calcium carbonate in the soil, which means that a portion of the soil is lost. Additionally, elemental sulfur or other acids must be added at low rates to prevent root damage to fruit crops. Applications must be made with care to avoid damage to the crop while trying to improve soil pH conditions for the same plant. One must consider that sulfate is left behind and may be leached to the water bodies and cause environmental risks, particularly in the Everglades area. Thus, a soil test is recommended before every planting or seeding of fruit crops.
In Florida, the majority of soils used for commercial fruit production are sandy. Sandy soils may need more frequent lime applications as compared to fine-textured soils (Havlin et al. 2005). Soil testing for soil pH and lime requirement is the best means for determining the need for an agricultural lime application, as well as the amount of lime to be applied (see EDIS publication UF/IFAS Standardized Fertilization Recommendations for Agronomic Crops, Mylavarapu et al. 2022).
The need for soil pH adjustment varies with fruit species. For example, mango may suffer from iron deficiency when soil pH is greater than 6.0. On the other hand, grape and peach can tolerate soils with a pH of 7.5 (Figure 1). For more information about adjusting soil pH, see EDIS publication HS1207, Soil pH Range for Optimum Commercial Vegetable Production (Liu and Hanlon 2022).
Fertilizer Programs
Fertilization, particularly macronutrient applications, can significantly adjust soil pH in the root zone instead of the whole plow layer. Nutrients can change soil pH differently depending on the electric charge of the nutrient ions. There are two types of nutrients: positively and negatively charged. The positively charged nutrients include ammonium-nitrogen, potassium, calcium, and magnesium. All of these nutrients may reduce soil pH in the root zone. The negatively charged nutrients include nitrate, phosphate, and sulfate. All of these nutrients may increase the pH in the root zone. Among the macronutrients, only nitrogen has two different forms with either positive or negative charges: ammonium-nitrogen or nitrate-nitrogen. After application, ammonium-nitrogen has two different fates: direct uptake by plant roots, or conversion to nitrates by soil bacteria and then absorption by plant roots. Both of these processes can significantly reduce soil pH. In direct uptake, plants release an equal amount of acid to the ammonium-nitrogen taken in. These released acids reduce the root zone soil pH significantly. This type of pH change may be the most effective way of modifying root zone pH for fertilized plants. Ammonium can produce twice as much acid as it is absorbed when it is first oxidized into nitrate in soil (Havlin et al. 2005). These produced acids reduce root zone soil pH when the fertilizer is applied and incorporated into the root zones.
Pine Bark Mulch
Pine bark mulch is acidic, with a pH of 3.7 to 4.0 (Wright et al. 1999). In high-pH soils, application of pine bark can lower soil pH over time. Blueberry prefers acidic growth conditions, so pine bark is usually used for blueberry orchards to serve as mulch and slowly lower soil pH. However, special attention needs to be paid to other crops, because continuous application of pine bark may make the soil too acidic for plants to grow. Producers need to consult county Extension agents when considering multiple applications of pine bark to the soil.
Peat Moss Mulch
Sphagnum peat moss (SPM) is acidic (pH 3.4–4.8) and can adjust soil pH from alkaline to neutral or slightly acidic. However, SPM is less economical than pine bark (Odneal and Kaps 1990).
Biochar
Chemically, biochar and charcoal are the same. Biochar is a name for charcoal when used as a soil amendment or for other special purposes, such as treatment of waste or polluted water. Just like charcoal, biochar is produced by pyrolysis of biomass using limited oxygen conditions. The pH of biochar depends on the production temperature. For example, at 662°F (350°C), the pH is approximately 4.0, but at 1,472°F (800°C), the pH may be as high as 11.0 (Gundale and DeLuca 2006). Low-temperature biochar can be used for reducing soil pH and high-temperature biochar for increasing soil pH.
The addition of lime, sulfur, biochar, pine bark, or peat moss to affect soil pH is always temporary and is a continuous, yet important, management issue. The natural soil pH will reoccur after one single or multiple growing seasons. The most economical and eco-friendly approach is, therefore, to match the natural soil pH and the fruit crop pH preference.
Summary
- Optimal soil pH can maximize fruit crop growth, development, productivity, and profitability.
- Florida soils used for fruit production have a wide pH range, from 3.6 to 8.4. Soil pH management is crucial for fruit production.
- Optimal soil pH varies with crops. Blueberry grows well with the soil pH less than 5.5, but peach is more productive at a pH slightly greater than 6.0.
- Soil pH management includes liming for low-pH soils and adding sulfur or acids to high-pH soils. The use of selected organic matter sources can also be effective for controlling soil pH with time.
- Fertilization program with different nitrogen fertilizers can effectively adjust root zone soil pH. Ammonium nitrogen may lower soil pH for fruit crops such as blueberry, but it is not effective with fruit crops requiring alkaline soil pH ranges, such as peach or black currant.
References
Burr, T. J., A. Caesar, and M. N. Schrolh. 1984. "Beneficial plant bacteria." 2 (1): 1–20.
Childers, N. F., J. R. Morris, and G. S. Sibbett. 1995. Modern Fruit Science: Orchard and Small Fruit Culture (10th Edition). Horticultural Publications, Gainesville, Florida.
Gundale, M. J., and T. H. DeLuca. 2006. "Temperature and substrate influence the chemical properties of charcoal in the ponderosa pine/Douglas-fir ecosystem." 231 (1–3): 86–93.
Havlin, J. L., J. D. Beaton, S. L. Tisdale, and W. L. Nelson. 2005. Soil Fertility and Fertilizers: An Introduction to Nutrient Management. Upper Saddle River, NJ: Pearson Education.
Herath, H. M. E. 1967. "Some effects of water table, pH, and ammonium and nitrate nitrogen upon the growth and composition of highbush blueberry." Thesis of Master of Science at the University of British Columbia.
Kering, M. K., and M. L. Kaps. 2004. "Soil pH affects growth and nutrient content of grapes." 39 (4): 827.
Liu, G. D., and E. Hanlon. 2022. Soil pH Range for Optimum Commercial Vegetable Production. HS1207. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/hs1207. Accessed on February 6, 2024.
Liu, G. D., E. Hanlon, and Y. C. Li. 2022. Soil pH Range for Optimum Commercial Vegetable Production. HS1208. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/hs1208. Accessed on February 6, 2024.
Mylavarapu, R. S., K. Hines, and T. Obreza. 2022. Diagnostic Nutrient Testing for Commercial Citrus in Florida. SL279. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/ss492. Accessed on February 6, 2024.
Mylavarapu, R. S., and W.D. Lee. 2022. UF/IFAS Nutrient Management Series: Soil Sampling Strategies for Precision Agriculture. SL190. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/ss402. Accessed on February 6, 2024.
Odneal, M. B., and M. L. Kaps. 1990. "Fresh and Aged Pine Bark as Soil Amendments for Establishment of Highbush Blueberry." Hort science 25 (10): 1228–1229.
Retamales, J. B., and J. F. Hancock. 2012. "Blueberries." Crop Production Science in Horticulture 21. Cambridge, MA: CABI.
Shober, A. L., C. Wiese, and G. C. Denny. 2022. Soil pH and the Home Landscape or Garden. SL 256. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/ss480. Accessed on February 6, 2024.
Splittstoesser, W. E. 1990. Vegetable Growing Handbook: Organic and Traditional Methods (3rd ed.). New York: Chapman & Hall.
Tagliavini, M., J. Abadía, A. D. Rombolà, A. Abadía, C. Tsipouridis, and B. Marangoni. 2008. "Agronomic means for the control of iron deficiency chlorosis in deciduous fruit trees." 23 (12-12): 2007–2022.
Tucker, D. P. H., J. S. Rogers, E. W. Stover, and M. R. Ziegler. 2007. Florida Citrus: A Comprehensive Guide. Gainesville: University of Florida Institute of Food and Agricultural Sciences.
USDA (United States Department of Agriculture). 1985. "Soil Survey of Alachua County Area, Florida." Natural Resources Conservation Service. Accessed July 16, 2013. https://ufdc.ufl.edu/UF00025129/00001 Accessed on February 6, 2024.
USDA (United States Department of Agriculture). 1989. "Soil Survey of Highlands County Area, Florida." Natural Resources Conservation Service. Accessed July 16, 2013. https://books.google.com/books/about/Soil_Survey_of_Highlands_County_Florida.html?id=qgQbwQEACAAJ Accessed on February 6, 2024.
USDA (United States Department of Agriculture). 1990a. "Soil Survey of Hendry County Area, Florida." Natural Resources Conservation Service. Accessed July 16, 2013. https://ufdc.ufl.edu/UF00025730/00001 Accessed on February 6, 2024.
USDA (United States Department of Agriculture). 1990b. "Soil Survey of Polk County Area, Florida." Natural Resources Conservation Service. Accessed July 16, 2013. https://ufdc.ufl.edu/UF00025726/00001 Accessed on February 6, 2024.
USDA (United States Department of Agriculture). 2017. " United States Department of Agriculture Handbook No. 18: Soil Survey Manual." https://www.nrcs.usda.gov/sites/default/files/2022-09/The-Soil-Survey-Manual.pdf Accessed on February 6, 2024.
Wright, A. N., A. X. Niemiera, J. R. Harris, and R. D. Wright. 1999. "Micronutrient Fertilization of Woody Seedlings Essential Regardless of Pine Bark pH." 17(2): 69–72.