Grafting as a cultural practice for controlling soilborne diseases and improving abiotic stress tolerance has been widely used in the production of tomato (Solanum lycopersicum L.), eggplant (Solanum melongena L.), pepper (Capsicum annuum L.), watermelon (Citrullus lanatus [Thunb.] Matsum. & Nakai), melon (Cucumis melo L.), and cucumber (Cucumis sativus L.) in many areas of Asia and Europe (Lee and Oda 2002; Lee et al. 2010; Louws, Rivard, and Kubota 2010). Interest in vegetable grafting has been growing in the United States in recent years, as well. By physically conjoining a plant with desirable fruit characteristics (called a scion) onto another plant with specific disease resistance or stress tolerance (called a rootstock), grafted plants combine the beneficial characteristics of both the rootstock and scion cultivars (Lee et al. 2010). Grafted plants, however, are more costly than the regular transplants (Barrett, Zhao, and Hodges 2012; Djidonou, Gao, and Zhao 2013). Cost, along with the desire to customize scion cultivars and the need to produce organic transplants, has led many small and organic growers to choose to graft plants by themselves. However, achieving a high graft survival rate often can be rather challenging for growers, especially during their initial attempts at grafting.
Different methods have been used commercially in grafting solanaceous and cucurbitaceous vegetable plants (Lee and Oda 2002; Lee et al. 2010). Although melon grafting follows the same principles as grafting other types of vegetables, it has some unique considerations. Firstly, both rootstock and scion plants develop hollow hypocotyls soon after seed germination, and this central cavity expands as plants grow. Hollow hypocotyls reduce the contact area between rootstock and scion tissues; therefore, grafting should be conducted with young seedlings when the central cavities are small. Secondly, in contrast to tomato grafting where scions can be cut above the cotyledons, melon scions are usually cut at the hypocotyl area, maintaining both cotyledons. Presence of scion cotyledons may result in greater leaf surface areas and cause water loss to be more severe with newly grafted melon plants than with tomato plants. Thirdly, rootstocks used for melon grafting can be intra- or interspecific and can have varied hypocotyl diameters. Grafting methods then need to be adjusted based on rootstock species and hypocotyl diameters.
Interspecific hybrid squashes (Cucurbita maxima Duchesne × Cucurbita moschata Duchesne) are widely used as melon rootstocks (King et al. 2010). They are highly resistant to Fusarium wilt and tolerant to Verticillium wilt, Monosporascus sudden wilt, and gummy stem blight (Guan et al. 2012; Louws, Rivard, and Kubota 2010). In addition, some interspecific hybrid squash rootstocks have tolerance to low temperatures and saline conditions (Colla et al. 2010; Davis et al. 2008). However, they are susceptible to root-knot nematodes and may have adverse effects on fruit quality of some melon cultivars (Davis et al. 2008; Guan et al., 2015; Sakata, Ohara, and Sugiyama 2008).
Melon cultivars with resistance to Fusarium oxysporum f. sp. melonis (race 0, 1, 2, and 1.2) are also used as rootstocks (Davis et al. 2008). The advantages of melons grafted onto melon rootstocks include fewer incompatibility issues and fewer fruit quality concerns. However, insufficient disease resistance is the major obstacle of using melon as a rootstock (King et al. 2010). African horned cucumber (Cucumis metulifer E. Mey. ex Naud.) was recently tested for melon grafting because it is resistant to root-knot nematode and fusarium wilt (Guan et al. 2014; Sigüenza et al. 2005; Trionfetti Nisini et al. 2002). While African horned cucumber is compatible with melon cultivars, it has thinner stems compared to melons, and special attention should be paid when it is used as a melon rootstock.
To help growers who are interested in grafting melon plants achieve a high graft survival rate, this article introduces commonly used grafting techniques and their application in specific circumstances.
Hole Insertion Grafting
The procedure for hole insertion grafting is demonstrated in Figure 1. First, true leaves and meristem tissue are removed at the growing tip of the rootstock. Next, a slit is made across the growing point from the bottom of one cotyledon to the other side of the hypocotyl. A shaved stick such as a toothpick or bamboo barbecue skewer can be used as the insertion tool. Leave the stick inserted in the growing point, while cutting the scion hypocotyl at both sides into a V shape. The scion is then inserted into the slit while the stick is removed.
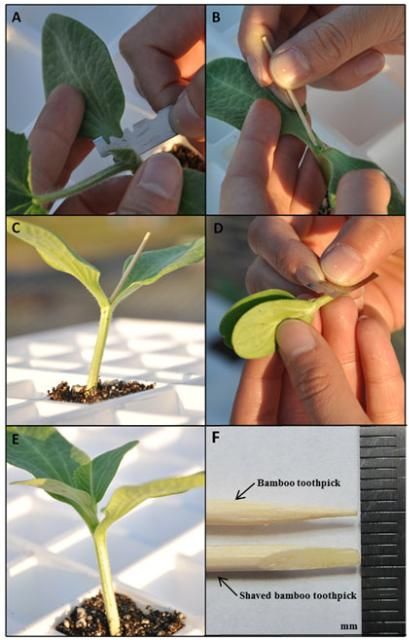
Credit: W. Guan, UF/IFAS
Hole insertion grafting produces high-quality grafted transplants because it may help increase the contacting surface area between rootstock and scion and provide protection of the graft union with both rootstock cotyledons. Another advantage of this method is that it does not require grafting clips, which reduces the grafting cost as well as the labor involved in collecting clips after healing.
Hole insertion grafting works best for hybrid squash rootstocks, as they normally have thicker hypocotyls than those of melon scions. Due to the concern of hollow hypocotyls, rootstocks with more than two expanded true leaves should be avoided. The ideal grafting period for scions is when the first true leaves start to emerge but are not fully expanded. During this stage, scion hypocotyls are strong enough to be inserted while still easy to fit into the slits in the rootstock. Since the range of ideal plant size for hole insertion grafting is relatively narrow, timing of sowing rootstock and scion seeds is critical for success. If a new rootstock or scion cultivar is used, it is recommended that the relative seed germination and growth rate of both rootstock and scion plants be determined before conducting large-scale grafting with this method.
Splice Grafting
The process of slice grafting is simpler than that of hole insertion grafting. The procedure for this method is shown in Figure 2. First, the rootstock growing tip is cut at a 45-degree angle. The cut removes true leaves, meristem tissue, and one of the cotyledons. Next, the hypocotyl of the scion is cut at the same angle as the rootstock. The scion is then attached to the rootstock with a grafting clip.
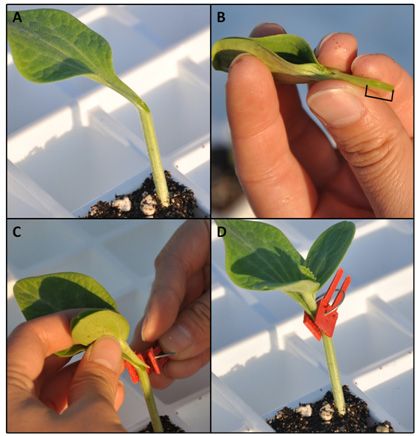
Credit: W. Guan, UF/IFAS
This method is most suitable for melon rootstocks, as it works best when the rootstock and scion have similar hypocotyl diameters. Hybrid squash rootstocks also are grafted with this method because it is simple to perform. However, since hybrid squash rootstocks generally have thicker hypocotyls compared to melon scions, scion seeds may be planted earlier than rootstock seeds. This method also can be applied to the African horned cucumber rootstock. In contrast to the hybrid squash rootstock, African horned cucumber has a thinner hypocotyl than melon, thus rootstock seeds should be sowed a few days earlier than scion seeds. Similar to hole insertion grafting, scions grafted at a younger stage have higher survivability due to reduced water loss. While young plants are desirable, a compromise may need to be made so that scion stem diameters do not differ greatly from those of rootstocks, particularly when hybrid squash rootstocks are used. It is important to note that grafting needs to be completed soon after plants are cut to prevent the surfaces of both scion and rootstock from drying out in order to improve the survival rate of grafted plants.
Tongue Approach Grafting
Tongue approach grafting is a popular grafting method used in Spain. Rootstock and scion seeds typically are sown in the same cell of the seedling tray, putting the hypocotyls of rootstock and scion plants close to each other. After they emerge, the rootstock hypocotyl is cut halfway through at a downward 45-degree angle. At the same height, the scion hypocotyl is cut halfway through at an upward 45-degree angle. The rootstock and scion plants then are joined together with the cut surfaces mated, and the hypocotyls are fixed with a grafting clip. Eight to ten days after grafting, the rootstock top and scion roots are cut off with a razor blade (Figure 3). Tongue approach grafting requires rootstock and scion to have similar hypocotyl diameters, so the timing of seeding rootstocks and scions should be adjusted based on their growth rate and hypocotyl diameters.

Credit: W. Guan, UF/IFAS
Since both rootstock and scion plants are grown for eight to ten days after grafting, this method may require larger greenhouse space and more resources during graft healing. In addition, tongue approach method can be rather challenging when rootstock and scion plants look quite similar, making it difficult to distinguish between rootstock and scion seedlings in the same planting cell. Moreover, some squash rootstocks have very short hypocotyls, which may not be suitable for this method.
Post-Graft Healing
With the hole insertion and splice grafting methods, maintaining high humidity and favorable temperatures during the first 48 hours after grafting is essential for graft success. The optimal temperature is around 85ºF and relative humidity is between 95 to 100 percent. Our experiments indicated that temperatures lower than 70ºF and/or average relative humidity lower than 85 percent in the first 48 hours could result in graft failure. If scions wilt severely during this period, their chance of recovery is very low. To reduce water loss through transpiration and evaporation, low light conditions may be required. Placing plants in darkness for longer than 48 hours, however, could result in unfavorable stem elongation and spindly seedlings. After the critical 48 hours, gradual reduction of humidity and an increase in the amount of light is important for graft healing and disease prevention. For small-scale grafting, storage containers may be used for post-graft healing (Figure 4). The container needs to be covered or placed under low light during the critical hours. High humidity can be maintained by putting a thin layer of water on the bottom and/or spraying water inside the closed container. After the critical hours, humidity is gradually reduced by partially opening the container while more light is introduced. More information regarding the construction of healing chambers can be found in Johnson et al. (2011). For larger-scale grafting, healing chambers that can accommodate several seedling trays can easily be constructed inside a greenhouse. For commercial post-graft healing, poly-covered healing structures inside greenhouses and germination or storage rooms with humidity and temperature control are normally used (Figure 5).

Credit: W. Guan, UF/IFAS

Credit: X. Zhao, UF/IFAS
Tongue approach grafting may achieve a high survival rate without the need for high-humidity healing conditions. Direct sunlight should be avoided on newly grafted plants, but healing often is acceptable in a normal greenhouse environment.
Rootstock Sucker Development
As a result of the incomplete removal of meristem tissue, re-growth of rootstocks—i.e., rootstock suckers—can be a potential issue when using hole insertion and splice grafting methods (Figure 6). Scouting and removing rootstock suckers should be conducted before field transplanting. However, suckers may continuously emerge in the field, which can become one of the major problems of using grafted transplants. Rootstock regrowth competes with scions for water and nutrients and reduces yield. Tongue approach grafting eliminates the rootstock sucker problem by completely removing the apical meristem of rootstocks.

Credit: W. Guan, UF/IFAS
In summary, each of the grafting methods has its own advantages and challenges. Decisions on choosing the most suitable method and technique to optimize production of grafted melon transplants will depend on available space, labor, and resources; rootstock and scion cultivars; the grafters' experience; and the ultimate economic considerations.
References
Barrett, C. E., X. Zhao, and A. W. Hodges. 2012. "Cost benefit analysis of using grafted transplants for root-knot nematode management in organic heirloom tomato production." HortTechnology 22:252–257.
Colla, G., Y. Rouphael, C. Leonardi, and Z. Bie. 2010. "Role of grafting in vegetable crops grown under saline conditions." Scientia Horticulturae 127:147–155.
Davis, A. R., P. Perkins-Veazie, Y. Sakata, S. López-Galarza, J. V. Maroto, S. G. Lee, Y. C. Huh, Z. Sun, A. Miguel, S. R. King, R. Cohen, and J. Lee. 2008. "Cucurbit grafting." Critical Reviews in Plant Sciences 27:50–74.
Djidonou, D., Z. Gao, and X. Zhao. 2013. "Economic analysis of grafted tomato production in sandy soils in Northern Florida." HortTechnology 23:613–621.
Guan, W., X. Zhao, R. Hassell, and J. Thies. 2012. "Defense mechanisms involved in disease resistance of grafted vegetables." HortScience 47:167–170.
Guan, W., X. Zhao, D. W. Dickson, M. L. Mendes, and J. Thies. 2014. "Root-knot nematode resistance, yield, and fruit quality of specialty melons grafted onto Cucumis metulifer." HortScience 49:1046–1051.
Guan, W., X. Zhao, D. J. Huber, and C. A. Sims. 2015. “Instrumental and sensory analyses of quality attributes of grafted specialty melons.” Journal of the Science of Food and Agriculture 95:2989–2995.
Johnson, S., C. Miles, P. Kreider, and J. Roozen. 2011. "Vegetable grafting: the healing chamber." Washington State University extension fact sheet. FS051E. 16 Feb. 2022. https://s3.wp.wsu.edu/uploads/sites/2071/2014/04/Grafting-Healing-Chamber-FS051E.pdf.
King, S. R., A. R. Davis, X. Zhang, and K. Crosby. 2010. "Genetics, breeding and selection of rootstocks for Solanaceae and Cucurbitaceae." Scientia Horticulturae 127:106–111.
Lee, J. M. and M. Oda. 2002. "Grafting of herbaceous vegetable and ornamental crops." Horticultural Reviews 28:61–124.
Lee, J. M., C. Kubota, S. J. Tsao, Z. Bie, P. Hoyos Echevarria, L. Morra, and M. Oda. 2010. "Current status of vegetable grafting: Diffusion, grafting techniques, automation." Scientia Horticulturae 127:93–105.
Louws, F. J., C. L. Rivard, and C. Kubota. 2010. "Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds." Scientia Horticulturae 127:127–146.
Sakata, Y., T. Ohara, and M. Sugiyama. 2008. "The history of melon and cucumber grafting in Japan." Acta Horticulturae 767:217–228.
Sigüenza, C., M. Schochow, T. Turini, and A. Ploeg. 2005. "Use of Cucumis metuliferus as a rootstock for melon to manage Meloidogyne incognita." Journal of Nematology 37:276–280.
Trionfetti Nisini, P., G. Colla, E. Granati, O. Temperini, P. Crinò, and F. Saccardo. 2002. "Rootstock resistance to fusarium wilt and effect on fruit yield and quality of two muskmelon cultivars." Scientia Horticulturae 93:281–288.