Currently in the Florida citrus industry, there is a high demand for replanting and resetting trees. There is also a growing interest in new releases of rootstocks and scions with improved tolerance to disease and abiotic stress. Consequently, more available plants are needed. Time is one of the main constraints for producing trees in a great number. The consensus among citrus nurseries is that it takes two years to produce the right quantity of budwood or to establish seed block trees when a new interesting rootstock is produced (Chaires 2017). In this chapter we address the practices and challenges to producing seeds and budwood in Florida.
1. Seed Production, Extraction, and Conservation
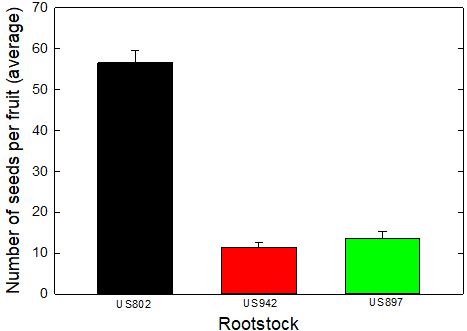
Rootstock propagation has traditionally depended upon production of clonal plants from nucellar seedlings to guarantee seed uniformity. HLB (huanglongbing, or citrus greening) plays a major role in all aspects of citrus production, including seed production. Fruit heavily infected by HLB may yield aborted seeds that will not germinate. Under the current situation of pressure for replanting and resetting HLB-affected groves, there is concern among citrus nursery operators and growers about seed availability of the most popular rootstocks in Florida. Currently, the supply does not satisfy industry demand of seeds. This disparity is aggravated by other factors, such as the fruit's incapability to produce a sufficient number of viable seeds (Figure 1) as well as the annual threat from hurricanes during late summer, which coincides with harvest time for the seed source fruit. Depending on the rootstock variety, hurricanes in August and September may reduce the amount of seed harvested by as much as 90%.
Availability and quality of rootstock seeds are among the most important factors in citrus liner production. Some nurseries have their own seed source trees, but other sources of seeds include the USDA and UF/IFAS, which contribute 4%–40% of seeds, depending on the rootstock. Seeds from California may account up to 50%–90% of seeds used (Chaires 2017).
Vigorous seeds are necessary to produce strong rootstocks. Seed vigor depends on the seed's level of maturation and can be influenced by the level of cold storage (Carvalho and Silva 2013). Cold storage (ideally 4°C) is imperative due to the biology of citrus. Under Florida conditions, seeds from many of the most popular rootstocks mature between July and October; therefore, it is imperative to store seeds in sufficient quantities to ensure continuous tree production throughout the year. Knowledge on the viability of seeds from different rootstocks is important to optimize timing of operations in the nursery. The precise maturation stage at which citrus seeds germinate best is unknown for most rootstock varieties. In general, the more mature a seed is, the longer it can be stored, but the length of time a seed can be stored and still be viable depends on seed maturity, and how these two parameters interact is not well known. The information currently available on the effects of fruit age on physiological responses of seedlings that germinated from seed obtained from fruit at different times of the season is scarce (Orbovic et al. 2011). Vigorous seed germination and seedlings with a well-defined root growth are necessary for the development of a healthy nursery stock; therefore, it is imperative that viable disease-free seeds be utilized to establish a nursery population that can result in vigorous scion growth and rapid establishment in the field.
Refrigeration, though necessary for optimal germination, can result in loss of seed viability if not done properly (Carvalho et al. 2005; Dantas 2009). Seed viability depends on seed vigor when under cold storage conditions. Seed vigor during storage is determined by a combination of factors, including moisture content, storage temperature (ideally 4°C), relative humidity, and carbon dioxide and oxygen pressures in the storage environment (Fawusi 1979). The effect of atmosphere on citrus seeds is not well understood; it seems that modified atmosphere does not have a clear beneficial effect on conservation. Seed moisture content and relative humidity are related parameters. Lower moisture levels inhibit seed germination, but higher moisture content may reduce storage life. Therefore, it is recommended to store seeds at low humidity conditions, although this varies among varieties. For example, Duncan grapefruit seeds will not germinate if moisture content is less than 6% (Orbovic et al. 2013), and other varieties will not tolerate any desiccation (reduction in water content). Once a seed is extracted and stored it may deteriorate. The deterioration rate of the seed is influenced by different factors, such as rootstock variety, humidity and air temperature, and contamination with pathogens (Koller et al. 1993; Martins et al. 2007). The fungi Aspergillus sp. and Penicillium sp. are among the most important pathogens and can grow at temperatures above 4°C. Desiccation improves storage of seeds, but some varieties, such as Swingle (Poncirus trifoliata × C. paradisi), require a higher water content for conservation, and their seeds generally have lower longevity (Carvalho, 2001).
To harvest the seeds, one must be sure that the fruit is fully mature, the signs of which depend on the variety. Avoid fruits that have dropped to the ground or that are located in the skirt section of the tree because they may carry Phytophthora spp. and other pathogens that could compromise seed longevity and viability. Seed extraction can be performed manually or mechanically, but mechanically is generally more efficient. After extraction, the seeds must undergo chemical treatment to remove mucilage (slime substance on the outer integument or "shell"). One efficient treatment for this used in the Brazilian nursery industry (Neto et al. 2016) consists of immersing the seeds in a solution containing hydrated lime (0.5%) for approximately 10 minutes. Then, seeds are washed in water and subjected to heat for 10 minutes at 125.6°F (52°C) in a solution containing 15 ml of caustic soda (NaOH) and 3 ml of hydrochloric acid per liter of seeds. The solution containing the seeds must be stirred every 15 minutes for 45 minutes. Later, the seeds are washed under running water and treated in a solution containing 100 g of hydrated lime to remove chemical residues. Finally, they are washed under running water while rubbed between the hands. After removal of the mucilage, the outer integument can also be removed at a temperature of 95°F–98.6°F (35°C–37°C) and pH between 11.5 and 12. The duration of this treatment may vary depending on the seed age and variety. According to Neto et al. (2016), storing seeds after the removal of the seed coat is not recommended because it may promote germination. Once the integument is removed from seeds, they should be promptly used or stored for a short time only. Seeds should be kept in plastic bags during cold storage. Seeds with the integument can be stored in a cold chamber for more than six months if maintained at a temperature of 39.2°F–46.4°F (4°C–8°C) and a relative humidity of 60%–80%. For more information on seed treatment and storage see chapter 6, HS1329 (Citrus Rootstock Propagation: Traditional Techniques and Recent Advances) of this guide.
2. Budwood Production
The scion of a citrus tree is the aboveground portion of the tree that produces the fruit. Scions come from budwood and are incorporated with the rootstock through grafting (budding). Grafting and budding are asexual methods of propagation, so the tree maintains the characteristics of the mother plant (budwood source). Budwood used for producing citrus nursery trees needs to be certified disease-free only. In Florida, the Citrus Budwood Registration Program was established in 1953 and became mandatory for all propagation of citrus in 1997. See https://www.fdacs.gov/Agriculture-Industry/Pests-and-Diseases/Plant-Pests-and-Diseases/Citrus-Health-Response-Program/Citrus-Budwood-Program for more information. The objective of this program is to assist growers and nurserymen in producing horticulturally superior citrus nursery stock free of graft-transmissible diseases and true to varietal type. Producing clean stock is the most effective way to avoid costly disease catastrophes in young plantings and their spread to other production areas.
Bud grafting, or budding, is a type of grafting in which the scion consists of a single bud. Budding works well for citrus and requires less skill than other types of grafting, so it is widely used for propagating citrus trees. See EDIS publication HS1309, Citrus Propagation (https://edis.ifas.ufl.edu/hs1309), for more information. Bud grafting can be conducted with one (single stem), two (double stem), or more branches (in stride). The conduction system will influence the spacing. A single-stem grafting allows less spacing between pots, which increases the number of plants. In addition, these plants are stronger, increasing the number of grafting buds per stem (Neto et al., 2016).
Shoots for budwood production should be young and dark green, flexible (should not break when bent), and healthy. After harvesting, budwood may dehydrate easily. To prevent dehydration and to facilitate manipulation, it must be transported quickly. In general, only the wood in the central part of the branch is used. When bud grafting is not performed immediately the branches may be refrigerated (5°C–10°C) in plastic bags for up to 2 months.
It is important to maintain budding and grafting tools in good, clean conditions. Viroids such as exocortis and cachexia can be spread from infected to healthy plants on clippers, budding knives, and other tools used in pruning and budding. For sterilization it is recommended to dip tools in a 20% solution of household bleach (1.05 sodium hypochlorite final concentration) for a few seconds before each use. Fresh solutions should be prepared daily because bleach rapidly loses its strength in the heat and sun. Tools can be stored or coated in spray oil or washed after use and then stored dry to prevent rusting.
References
Carvalho, J. A. 2001. Conservação de sementes de citros. 140f. Tese (Doutorado em Agronomia) Escola Superior de Agricultura de Lavras.
Carvalho, S. A., C. C. D. Graf, and A. R. Violante. "Produção de material básico e propagação." In: Mattos Jr., D., J. D. De Negri, R. M. Pio, and J. Pompeu Jr. Citros. Campinas: Instituto Agronômico e Fundag, 2005. cap. 10, p. 279–31.
Carvalho, S. A., and L. F. C. Silva. 2013. "Monitoring the Viability of Citrus Rootstocks Seeds Stored under Refrigeration." Revista Brasileira de Fruticultura. 35(1): 238–245.
Chaires, P. 2017. "Citrus Nurseries Analyze the Need for Seed." Growing Produce. Feb 22.
Chaires, P. 2018. "Clean Citrus Budwood the Product of Solid Team Effort." Growing Produce. Apr 12.
Dantas, I. B. 2009. Condicionamento fisiológico em sementes de limão-cravo e citrumelo Swingle. 123f. Dissertação (Mestrado em Agronomia) - Universidade Federal de Lavras, Lavras, 2009.
Fawusi, M. O. A. 1979. "Viability of Citrus Seeds as Influenced by the Methods of Drying and Storage Temperatures." Nigerian J. Sci. 13:313–322.
Koller, O. L., H. Stuker, and L. F. Verona. 1993. "Efeito da umidade, temperatura de estocagem e duração da estocagem sobre a germinação de Poncirus trifoliatae de outros porta-enxertos de cítrus." Revista Brasileira de Fruticultura, Jaboticabal. 15(1): 27–33.
Martins, L., W. R. Silva, and A. A. Lago. 2007. "Conservação de sementes de tangerina 'cleópatra': teor de água e temperatura do ambiente." Revista Brasileira de Sementes, Londrina. 29(1): 178–185.
Neto, H. B., S. R. da Silva, F. Alves Mourão Filho, M. B. Sposito, and M. M. Caputo. 2016. The Citrus Nursery Practices in Brazil. Araraquara: Vivecitrus Organização Paulista de Viveiros de Mudas Cítricas.
Orbovic, V., M. Dutt, and J. W. Grosser. 2011. "Seasonal Effects of Fruit Age on Regeneration Potential and Transformation Success Rate in Three Citrus Cultivars." Scientia Horticulturae 127: 262–266.
Orbovic, V., M. Dutt, and J. W. Grosser. 2013. Evaluation of the Germination Potential of Citrus Seeds during the Harvesting Season. Hortsci. 48: 1197–1199.