Introduction
The grass carp, Ctenopharyngodon idella Cuvier and Valenciennes, was imported to the United States in 1963 as a biological control agent for hydrilla (Hydrilla verticilliata (L.f.) Royle) and other aquatic plants. Efficacy experiments were conducted in Florida in the 1970s by the United States Department of Agriculture and the University of Florida. Use of the fish was limited from 1970 until 1984 due to tight regulations surrounding concerns of escape and reproduction, and the potential impacts that colonization of the fish could have on native flora and fauna. These concerns led to research that developed a non-reproductive fish, which was equally effective in controlling hydrilla.
Sterile fish were developed by subjecting eggs to stress, such as heat stress (hot or cold) or pressure. The stress causes each egg to retain an extra set of chromosomes and become triploid instead of diploid. Although triploid fish are virtually sterile, this does not affect their aquatic plant herbivory. Concern over the success rate of the sterilization technique led to screening for diploid individuals by measuring the diameter of cell nuclei, as triploid cells have larger nuclei. In the warm waters of Florida, with abundant food, grass carp grow quickly at around 2 lb/month or 0.91 kg/month and may achieve weights of 97 lb (44 kg) (Sutton et al. 2012). Younger fish and female fish grow faster than older or male fish.
The introduction of grass carp is the most effective biological control tool that has been identified for hydrilla. Additionally, although conversion of plant material to protein by the grass carp is not highly effective, it is still the best use for hydrilla. Every 1 lb (0.45 kg) increase in fish weight requires 5–6 lb (2.3–2.7 kg) of dry hydrilla (Sutton et al. 2012), which—considering hydrilla is 95% water—is a great deal of live plant material.
Synonymy
Leuciscus idella Cuvier and Valenciennes 1844
Leuciscus tschiliensis Basilewsky 1855
Ctenopharyngodon laticeps Steindachner 1866
Sarcocheilichthys teretiusculus Kner 1867
Ctenopharyngodon idellus Günther 1868
Pristiodon siemionovi Dybovskii 1877
(According to Shireman and Smith 1983)
Distribution
The grass carp is native to rivers that feed into the Pacific Ocean in eastern Russia and China, but it has been introduced to 70 countries including the United States, Taiwan, Japan, Mexico, India, Malaysia, and several European countries. In the United States, grass carp are so effective for weed control that they are used nationwide. In 2009, the use of grass carp was recorded in all states except Alaska, Maine, Montana, Rhode Island, and Vermont. Within the native range of the grass carp, the natural habitat includes low-gradient, large turbid rivers and associated lakes. Grass carp are highly temperature tolerant, and their native range includes both cold and warm water environments. Early release of diploid fish led to reproductive populations in several US drainage systems, including the Mississippi River and major tributaries.
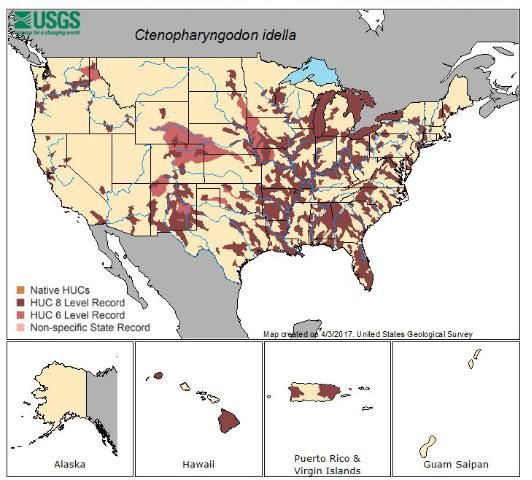
Credit: Map reproduced with permission from NAS
Within the United States, the distribution in water bodies is widespread, particularly in the Mississippi River basin and southeastern states. In Figure 1, distribution of the grass carp is classified by drainage system at two scales, fine and medium. Medium scale or HUC 6 is known as a basin and is on average 10,600 square miles in area. Fine scale or HUC 8 is known as a sub-basin and is on average 700 square miles in area. Occurrence of grass carp within a basin or sub-basin results in highlighting the entire drainage system. Drainages with reproductive, established populations are much less prevalent than suggested by the overall distribution of stocked and reported grass carp shown in Figure 1, many of which are non-reproductive triploids. Established populations occur in the Mississippi River basin and some drainages of eastern Texas.
Description
Eggs
Unfertilized eggs are 1.2–1.3 mm in diameter and have a yolk surrounded by a double-layered membrane (Shireman and Smith 1983; Figure 2). The outer layer is adhesive until fertilization (Shireman and Smith 1983). Fertilized eggs are 3.8–4.0 mm in diameter, and the yolk is separated from the membrane by water that is absorbed (Shireman and Smith 1983). Spawn containing eggs can be grayish-blue to bright orange (Shireman and Smith 1983).

Credit: Reuben Goforth, Purdue University
Protolarvae (Days 1–3)
Protolarvae hatch from the eggs at 5.0–5.5 mm in length (Figure 3). At this stage, they are transparent and completely without pigment. Within three days, they grow to 7.4–7.5 mm and develop useable gills. At this stage, the eyes become pigmented with gold irises, and the head and dorsum are green/yellow. During this time, protolarvae also begin to swim. Although protolarvae still are feeding mainly from the yolk sac, from day 2, the larvae will start to eat algae.
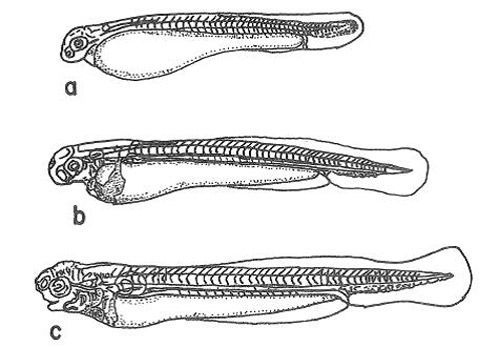
Credit: Shireman and Smith (1983) and used with permission from the Food and Agriculture Organization of the United Nations
Mesolarvae (Days 4–20)
By day 4, the larvae are 7.5–8.0 mm with a functional swim bladder and gills (Figure 4). The larvae become more motile and more pigmented every day. By day 20, the mesolarvae are 11.5–18.6 mm, and the fins have formed. The larvae are highly pigmented with a brown/yellow dorsum fading to white at the belly. As the yolk sac is quickly depleting, the larvae start to feed from the environment on algae and zooplankton, and by day 5 feed almost exclusively on zooplankton.
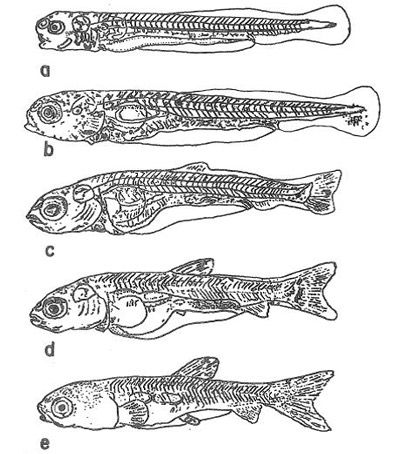
Credit: Shireman and Smith (1983) and used with permission from the Food and Agriculture Organization of the United Nations
Fry (Days 20–30)
Fry are 1.5–2.3 cm with well-developed fins and scales (Figure 5a). The teeth have formed, and the jaw has set. The swim bladder and the intestine resemble those of an adult. Fry feed on zooplankton and aquatic insect larvae. At 2 cm in length, the fry begin to eat aquatic plants.
Fingerlings (Days 45–60)
Fingerlings are 3.7–6.7 cm in length and resemble small adults (Figure 5b). By day 50, the scales are complete, and at approximately day 55 and 6.7 cm in length, the fingerling is identical to an adult. Fingerlings can eat animal food (e.g., insects and zooplankton), but by 5.5 cm in length are eating mainly plants.
Credit: Shireman and Smith (1983) and used with permission from the Food and Agriculture Organization of the United Nations
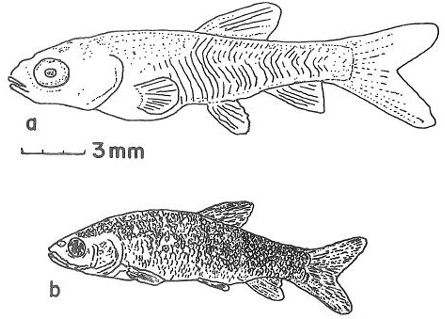
Juveniles (1–9 years)
Juveniles continue to grow and develop, but they already look identical to adults (Figure 6). The body of a juvenile or adult grass carp is torpedo shaped. The mouth angles downwards and the lips are firm and lacking barbells (i.e., fleshy whiskers). The body is dark olive in color, with brown to yellow shading on the sides and a white underside. The scales are large and outlined in brown, and the complete lateral line has 40 to 42 scales. Compared to other cyprinids, the anal fin is relatively close to the tail fin. Juveniles can feed on animal food (e.g., insects and zooplankton), but like adults, prefer to feed on plants. As the fish get larger and older, they feed on tougher plants of greater variety.

Credit: Jeffrey E. Hill, University of Florida
Adults
The maximum length of a grass carp is 4.6 ft (1.4 m), and the maximum weight is 97 lb (44 kg). Adults look identical to juveniles (Figure 7). Adult grass carp prefer to eat hydrilla compared to all other aquatic plants.

Credit: Jeffrey E. Hill, University of Florida
Life Cycle
Although the grass carp is highly adaptable and can survive in a variety of conditions, the natural grass carp life cycle has not been observed to occur many times outside of the native range. The restriction is related to reproduction, as the fish cannot reproduce in confined water bodies. The status of introduced grass carp populations is often difficult to determine because stocked individuals live such a long time and frequently there is little monitoring for successful recruitment. Of all the countries where the fish were introduced, they have established primarily in a few countries in Asia and Europe (Shireman and Smith 1983; Froese and Pauly 2017). However, there have been reports of several other sites having breeding populations including the Atchafalaya, Mississippi (and major tributaries), and the Trinity rivers in the United States (Shireman and Smith 1983; Nico et al. 2017).
In native areas, adult grass carp spawn in long fast-moving rivers at temperatures of 68–86°F (20–30°C). Spawning is triggered by increases in flow rate and temperature. Spawning generally occurs at the surface and is usually promiscuous, involving many males to each female (Shireman and Smith 1983). Fertilization occurs externally, and the semi-buoyant eggs then develop in the water column and may drift 30–100 miles (50–180 km) before hatching (Shireman and Smith 1983). Each female lays 500,000 eggs per brood on average, and fecundity increases with age (Shireman and Smith 1983). However, most eggs are lost to suffocation, disease, or predation (Shireman and Smith 1983). If the water temperature surrounding the eggs drops below 64°F or 18°C, the hatch rate and survival of larvae will be low (Shireman and Smith 1983).
Larvae have a characteristic movement that involves alternating between swimming and sinking. These larvae migrate from fast-moving rivers into lakes that act as nurseries for the juvenile fish. As juveniles, they migrate up- or downstream and spend the winter in deep holes in the riverbed (Shireman and Smith 1983). Juvenile grass carp feed on small invertebrates but shift to a plant-based diet by the time they reach 2 inches (5 cm) in length (Colle 2009). Female grass carp mature at 23–26 inches (58–67 cm) and males approximately one year earlier at 20–24 inches (51–60 cm). The average life of a grass carp is from 5 to 9 years. However, a grass carp may live for 20 years or more (Sutton et al. 2012).
Outside of most native areas, and for the cultivation of grass carp in the United States for aquatic plant management, fertilization is completed artificially. Sexually mature male and female fish are injected with hormones to promote ovulation and sperm production (Shireman and Smith 1983). Sperm, which are collected from the males, and eggs from the females are mixed and incubated with aeration to maintain movement of the eggs as they would experience in a fast-moving river.
Targeted Aquatic Plants
The grass carp is a grazer, feeding on vegetation mostly near the surface and in shallower waters. The new growth of submersed plants is preferred. Plant feeding preference is dependent on fish size, with small fish preferring musk grass (Chara spp.) and large fish preferring hydrilla (Sutton et al. 2012, Sun et al. 2017). Plant preference appeared to follow a trend towards softer plant material with lower levels of plant secondary metabolites (Sun et al. 2017). However, the grass carp is a generalist, and in the absence of the preferred plant, will feed on most other types of aquatic vegetation. Grass carp even have been observed to feed on terrestrial plants that are hanging over the water. The five most-preferred species in order of preference are hydrilla, musk grass, pondweeds (Potamogeton spp.), southern naiad (Najas guadalupensis [Spreng] Magnus), and Brazilian elodea (Egeria densa Planch Anderson) (Sutton et al. 2012). Grass carp is not a good control method for filamentous algae, Eurasian milfoil (Myriophyllum spicatum L.), spatterdock (Nuphar advena Aiton), fragrant waterlily (Nymphaea odorata Aiton), sedge (Cladium spp.), cattail (Typha spp.), or other large aquatic plants (Colle 2009).
Plant Consumption
Grass carp lack teeth in their jaws but have comb-like teeth on their pharyngeal arches (located in the throat) that enable them to grind vegetation. In fact, their scientific name means "distinctive comb pharyngeal teeth." Small fish will eat only the leaves, but as they increase in size, they will eat both leaves and stems (Edwards 1974). As adults, they consume large amounts of plant material, preferentially hydrilla. In suitably warm water (68°F or 20°C), an adult grass carp will consume its body weight in hydrilla every day (Edwards 1974). Although adult grass carp consume a lot of plant material, the conversion to animal protein is limited. For a 1 lb (0.45 kg) increase in fish weight, the fish must eat the equivalent of 5–6 lb (2.3–2.7 kg) of dry hydrilla (Sutton et al. 2012).
Stocking Rates
To ensure that hydrilla consumption by the fish exceeds the growth rate of the plant, several factors need to be considered, including age and sex of the fish. Depending upon these factors and the type, abundance and location of the plants within the water body, a stocking density can be determined. A study that investigated the effect of stocking rates on the ecosystem in 38 lakes in Florida found that 25 to 30 grass carp per hectare vegetation was the rate that produced the best control while leaving some plant species less preferred by the carp (Hanlon et al. 2000). In the study, this was equivalent to 10 to 15 grass carp per hectare of lake area (Hanlon et al. 2000). Of the 38 lakes, 27 had a hydrilla problem (Hanlon et al. 2000). Stocking rates greater than 30 grass carp per hectare vegetation resulted in complete removal of all vegetation and rates of less than 25 grass carp per hectare vegetation resulted in insufficient control of the target plant (Hanlon et al. 2000). The Florida Fish and Wildlife Conservation Commission typically recommend stocking 7.5 to 30 fish per hectare of lake area (3 to 10 fish per acre).
Ecosystem Effects
An ecosystem that has been stocked with grass carp will change in several ways if the aquatic vegetation is eliminated. Phytoplankton (small floating aquatic plants) will increase and cause a decrease in water clarity (Colle 2009). Fish species that are reliant on vegetation (e.g., chain pickerel, bluespotted sunfish, and golden topminnow) will decline and may be eliminated from the ecosystem, and species that feed on phytoplankton (e.g., gizzard shad and threadfin shad) will increase in number. This species composition change has occurred in several lakes in Florida that were stocked with grass carp (Colle and Shireman 1994).
Importance as a Biological Control Agent
Several studies have demonstrated the effectiveness of grass carp for aquatic plant management (Figure 8, Colle and Shireman 1994, Manuel et al. 2013). In two lakes in Florida, hydrilla infestations were eliminated in 4–5 years (Colle and Shireman 1994). In five other lakes in Florida, submersed aquatic plants were removed successfully in 1970 and remained controlled for at least 20 years (Colle and Shireman 1994). In four out of five Piedmont reservoirs, combining grass carp with herbicide treatments provided quick (within one year) and long-lasting control (Manuel et al. 2013).

Credit: David Sutton, University of Florida
An integrated program utilizing grass carp will be more cost effective than herbicide treatments alone. In 1994, a study estimated that, over a 9-year management program (1986–1994), the use of grass carp saved $200,000 (Jaggers 1994). The Florida Fish and Wildlife Conservation Commission state on their website that grass carp may cost $15 to $150 per acre depending on price and stocking rate, herbicides can cost $100 to $500 per acre, and mechanical control around $1,000 per acre. Additionally, while grass carp will continue to provide control, both chemical and mechanical control will need to be continuously implemented.
When introduction of a biological control agent is considered, the first condition that needs to be met is usually host specificity. Although large adult grass carp prefer hydrilla, younger smaller individuals prefer other plants. Furthermore, when hydrilla has been removed from the lake, the carp will eat other less-preferred plants. Therefore, it is important that lakes are not overstocked because the fish are difficult to remove once introduced. Some native plant species are less sensitive to grass carp defoliation and can establish even at medium to high stocking rates, in particular Nymphaea odorata, Echinodorus cordifolius, Justica americana and Pontederia cordata (Dick et al. 2016). These plants could be useful in native plant restoration, although the presence of other herbivores will need to be considered.
Grass carp must only be stocked into closed water bodies. In open water bodies, any canals, channels, or streams leading into other areas must be blocked with barriers to prevent fish escape. The barriers need to have a fine enough mesh to prevent the smallest fish from swimming through and must be high enough so that the fish cannot jump over.
Small grass carp may be lost to predation by birds, snakes, and other species of fish. In water bodies with largemouth bass, it is recommended to stock fish larger than 12 inches (30 cm) or 1 lb (0.45 kg).
Every state has different regulations for the use of grass carp. Florida does not permit diploid grass carp, but some states such as Alabama allow diploid fish. Florida permits the release of triploid grass carp, but some states do not allow triploids (e.g., Maryland), and some states such as Michigan have banned the release of any grass carp. Florida requires that the released fish are certified triploid and that a permit is obtained for use, possession, and removal of grass carp. Permits can be obtained from the Florida Fish and Wildlife Conservation Commission.
Monitoring and Management
Grass carp monitoring could be completed by netting or electrofishing along transects or by using hydroacoustics (Baerwaldt et al. 2013). Hydroacoustic techniques are non-invasive but do not identify fish to species. However, grass carp are rarely monitored once released.
When stocking grass carp, consider that they may eventually need to be removed once control of the aquatic weeds have been achieved. Removal is not easy without killing all fish in the water body and requires a permit. Several methods have been tested without much success—particularly in large water bodies—including netting, electrofishing, and rotenone treatments (Colle and Shireman 1994). Removal is usually a slow process through predation, fishing, and natural mortality. Fishing can be particularly effective in small systems.
The authors would like to acknowledge funding provided by the USDA NIFA RAMP Grant 2010-02825 that helped pay for the production of this article. The authors would like to acknowledge the reviewers that provided feedback on an early draft of the article, Dr. Chuck Cichra and Dr. Verena Lietze.
Selected References
Baerwaldt K, Herleth-King S, Shanks M, Monroe E, Simmonds R, Finney S, Stewart J, Parker A, Bloomfield N, Hill T, Doyle W, Morrison S, Santucci V, Irons K, McClelland M, O'Hara M, Wyffels D, Widloe T, Caputo B, Ruebush B, Zeigler J, Gaikowski M, Glover D, Garvey J, Freedman J, Butler S, Diana M, Wahl D. 2013. Monitoring and response plan for Asian carp in the upper Illinois river and Chicago area waterway system. [E1] Asian Carp Regional Coordinating Committee Monitoring and Response Workgroup, 152 pp. (24 August 2022).
Colle D. 2009. Grass carp for biocontrol of aquatic weeds. 61–64 pp. In Gettys LA, Haller WT, Bellaud M (editors). Biology and control of aquatic plants: A best management practices handbook. Aquatic Ecosystem Restoration Foundation, Marietta, Georgia.
Colle DE, Shireman JV. 1994. Use of grass carp in two Florida laeks, 1975-1994. In Proceedings of the grass carp symposium, U.S. Army Corps of Engineers, Vicksburg, MS. (22 April 2020).
Dick GO, Smith DH, Schad AN, Owens CS. 2016. Native aquatic vegetation establishment in the presence of triploid grass carp. Lake and Reservoir Management 32: 225–233.
Edwards DJ. 1974. Weed preference and growth of young grass carp in New Zealand. New Zealand Journal of Marine and Freshwater Research 8: 341–350.
Florida Fish and Wildlife Conservation Commission. 2014. Triploid grass carp permit: Are grass carp the answer? Florida Fish and Wildlife Conservation Commission. (24 August 2022).
Froese R, Pauly D. Editors. 2017. FishBase. Ctenopharyngodon idella (Valenciennes, 1844) Grass carp. World Wide Web electronic publication. (22 April 2020).
Jaggers BV. 1994. Economic considerations of integrated hydrilla management: a case history of Johns Lake, Florida. In Proceedings of the grass carp symposium, U.S. Army Corps of Engineers, Vicksburg, MS. (22 April 2020).
Hanlon SG, Hoyer MV, Cichra CE, Canfield DE. 2000. Evaluation of macrophyte control in 38 Florida lakes using triploid grass carp. Journal of Aquatic Plant Management 38: 48–54.
Manuel KL, Kirk JP, Barwick DH, Bowen TW. 2013. Hydrilla management in Piedmont reservoirs using herbicides and triploid grass carp: a case study. North American Journal of Fisheries Management 33: 488–492.
Nico LG, Fuller PL, Schofield PJ, Neilson ME, Benson AJ, Li J. 2020. Ctenopharyngodon idella (Valenciennes in Cuvier and Valenciennes, 1844). US Geological Survey Nonindigenous Aquatic Species Database, Gainesville, FL. Revision Date: 1/5/2020, Peer Review Date: 2/2/2016 (22 April 2020).
Pípalová I. 2006. A review of grass carp use for aquatic weed control and its impact on water bodies. Journal of Aquatic Plant Management 44: 1–12.
Shireman JV, Smith CR. 1983. Synopsis of biological data on the grass carp Ctenopharygngodon idella (Cuvier and Valenciennes, 1844). Food and Agricultural Organization of the United States. FAO Fisheries Synopsis No. 135 fir/s135 SAST - Grass carp - 1,40(02)035,01. (22 April 2020).
Sun J, Wang L, Ma L, Min F, Huang T, Zhang Y, Wu Z, He F. 2017. Factors affecting palatability of four submerged macrophytes for grass carp, Ctenopharyngodon idella. Environmental Science and Pollution Research 24: 28046–28054.
Sutton DL, Vandiver VV, Hill J. 2012. Grass Carp: A fish for Biological Management of Hydrilla and Other Aquatic Weeds in Florida. BUL867. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://doi.org/10.32473/edis-fa043-2012 (24 August 2022).