Introduction
Many species of thrips can be found in Florida. These include adventive species like Frankliniella occidentalis, Frankliniella schultzei, Thrips palmi, and Scirtothrips dorsalis. Native species include Frankliniella tritici and Frankliniella bispinosa. Frankliniella occidentalis is a pest of several crops throughout Florida and the world and is capable of causing economic loss (Fig. 1).

Credit: Lyle Buss
Taxonomy
The order Thysanoptera consists of more than 5,000 species in two suborders, Tubulifera and Terebrantia. The suborder Tubulifera has over 3,000 species in one family, Phlaeothripidae. The suborder Terebrantia consists of over 2,000 species in seven families. Thripidae is the largest of these families, with about 1,700 species. It includes genera such as Scirtothrips, Thrips, and Frankliniella (Mound and Teulon 1995; Mound et al. 2009).
Synonyms
The original name for Frankliniella occidentalis was Euthrips occidentalis Pergande 1895 (Hoddle et al. 2012; GBIF 2014). This species has a high number of synonymies as a result of the variability that Frankliniella occidentalis has in structure and color in its native range.
Some other synonyms are (CABI 2014):
Euthrips helianthi Moulton 1911
Euthrips tritici var. californicus Moulton 1911
Frankliniella californica Moulton
Frankliniella tritici var. moultoni Hood 1914
Frankliniella canadensis Morgan 1925
Frankliniella nubila Treherne 1924
Frankliniella chrysanthemi Kurosawa 1941
Frankliniella claripennis Morgan 1925
Frankliniella conspicua Moulton 1936
Frankliniella treherni Morgan
Frankliniella dahliae Moulton 1948
Frankliniella tritici maculata Priesner 1925
Frankliniella dianthi Moulton 1948
Frankliniella occidentalis f. brunnescens Priesner 1932
Frankliniella moultoni Hood
Frankliniella occidentalis f. dubia Priesner 1932
Frankliniella syringae Moulton 1948
Frankliniella venusta Moulton 1936
Frankliniella umbrosa Moulton 1948
The Entomological Society of America (ESA)-approved common name for this species is western flower thrips. Other common names around the world include alfalfa thrips, trips occidental de las flores, trips de California, thrips californien, thrips des petits fruits, trips del maiz, and kalifornischer blütenthrips.
Distribution
Western flower thrips is a native of western North America. It remained confined to western North America (west of 100°W longitude) until the 1960s. In the following decades it has spread with the horticultural trade throughout North America and the world (Kirk and Terry 2003; CABI 2014). According to CABI (2014), its current distribution includes:
Asia: China, Iran, Japan, Republic of Korea, Kuwait, Malaysia, and Sri Lanka
Africa: Algeria, Kenya, Morocco, Reunion, Swaziland, Tunisia, Uganda, and Zimbabwe
North America: Canada, Mexico, and the United States of America
Central America: Costa Rica and Guatemala
South America and the Caribbean: Dominican Republic, Martinique, Puerto Rico, Argentina, Brazil, Ecuador, Chile, Colombia, Guyana, Peru, Uruguay, and Venezuela
Europe and the Mediterranean: Albania, Austria, Belgium, Bosnia-Herzegovina, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Guernsey, Hungary, Ireland, Israel, Italy, Latvia, Malta, Montenegro, the Netherlands, Norway, Poland, Portugal, Romania, Russia, Serbia, Slovakia, Slovenia, Spain, Sweden, Turkey, the United Kingdom, and Ukraine
Oceania: Australia and New Zealand
In cooler areas western flower thrips may not overwinter or may be restricted to greenhouses. In Florida it is found throughout the state but it is more predominant in the north (Salguero-Navas et al. 1991; Childers and Beshear 1992; Kirk and Terry 2003).
Description
Eggs: The eggs are small (550 µm X 250 µm) and oval-shaped (Lewis 1973 as cited by Mau and Martin; CABI 2014). The eggs of thrips in the suborder Terebrantia are inserted into any non-lignified tissue of a plant (Reitz et al. 2011; CABI 2014).
Larva I and II: Larvae are small and wingless and may be found in flowers (Fig. 2), buds, or places where leaves are touching. These are actively feeding stages. (Reitz 2009; CABI 2014).

Credit: Lyle Buss
Prepupa and pupa: The prepupa is characterized by short wing buds and antennae that are not pulled back over the head (Fig. 3). The pupae have longer wing buds, and the antennae are pulled backward over the head. These non-feeding life stages usually are passed in soil, although they can occur in complex flowers like chrysanthemum. (CABI 2014)

Credit: Lyle Buss
Adults: Adults have fully developed wings with long fringes of cilia typical of most Thysanoptera. The adult of this species is less than 2 mm in length. It has three color morphs. These color morphs are termed dark-brown, light, and intermediate (yellow with a dark longitudinal band along the dorsum of the thorax and the abdomen). The intermediate morph is the most common in Florida. Males usually make up a much smaller proportion of the population and are smaller and paler than females. Adults and larvae may be found in similar locations (Reitz 2009). The cryptic behavior of western flower thrips limits exposure to insecticides (Brodsgaard 1994).
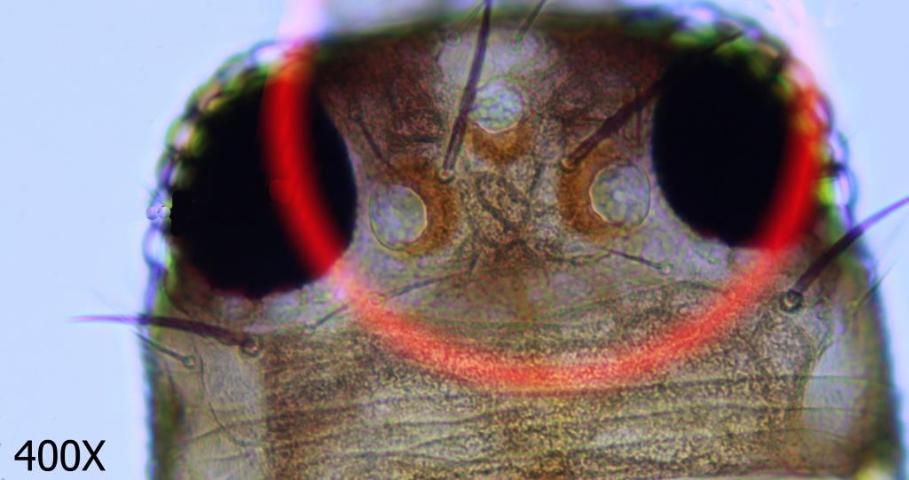
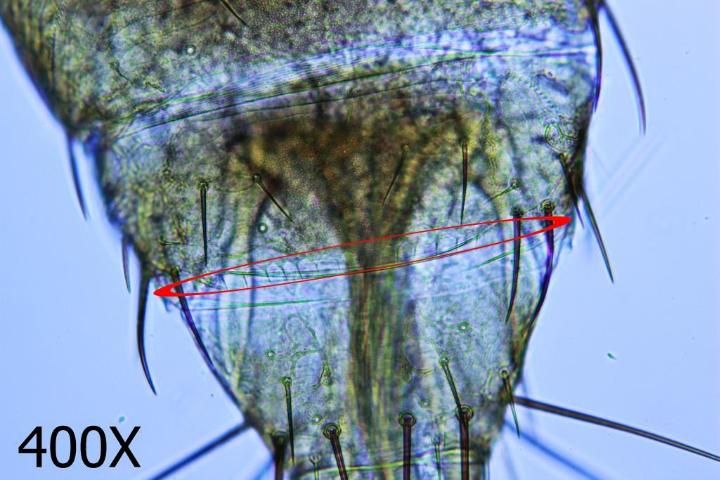
This species may be differentiated from its congers by key diagnostic features. These include a smooth antennal pedicel (Fig. 4), spines arising from the second antennal segment that are not exceptionally heavy (Fig. 5), a pair of ocular setae that are separated by at least one and a half times the diameter of a single ocellus (Fig. 6), four small setae arising on the anterior margin of the prothorax between the major antemarginal setae (Fig. 7), and a microtrichial comb on abdominal segment VIII that is complete and well-developed (Fig. 8).

Credit: Jeff Cluever
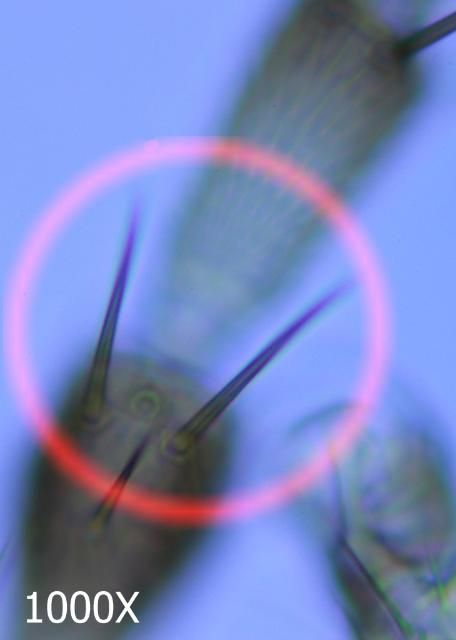
Credit: Jeff Cluever
Credit: Jeff Cluever

Credit: Jeff Cluever
Credit: Jeff Cluever
Biology and Behavior
Western flower thrips has a punching-sucking feeding habit, using the mandible to punch a hole into the host and then inserting the maxillae into the opening. The maxillae then ingest the fluids from the cells, but not directly from the vascular tissue. Western flower thrips will also ingest the contents of pollen grains. There is evidence of predatory behavior in this species. In cotton this species was found to be a predator of spider mite eggs (Gonzalez and Wilson 1982, Hunter and Ullman 1989, Kindt et al. 2003).
Western flower thrips males are produced from unfertilized eggs and females are produced from fertilized eggs. The adult female uses her saw-like ovipositor to oviposit (lay eggs) into foliage of a plant. Even though this species does not exhibit sociality, males will form mating swarms (Terry and Gardener 1990, Lewis 1991a).
Western flower thrips individuals are not strong fliers, but the adults are capable of dispersal over long distances (Ramachandran et al. 2001). The fringed posterior surface of the wings, typical of most thrips species, enhances their ability to fly. There is a record of the thrips species Frankliniella tritici and Haplothrips graminis being caught on aircraft-mounted sticky traps at 10,000 feet (Glick 1939 as cited by Lewis 1991b, Lewis 1991b).
Life history
On cabbage leaves at 27°C, western flower thrips develops from egg to adult in 10.2 days. It takes western flower thrips 3.07, 1.78, 2.38, 1.00, and 2.04 days to complete the egg, larva I, larva II, prepupal, and pupal stages respectively. At this temperature, an adult female produces an average of 76.6 eggs in her lifetime (Zhang et al. 2011).
Hosts
Western flower thrips is a polyphagous species with hosts in 65 families (CABI 2014). Some examples of host plants are alfalfa, apricots, artichoke, carnations, chrysanthemum, corn, cotton, cucumber, eggplant, gerbera, gladiolus, grapefruit, grapes, impatiens, melons, nectarines, peaches, peanut, peas, pepper, plums, Spanish needle, strawberry, tomato, watermelon, and wild radish. Note that a species classified as a host still may not be able to support reproduction of western flower thrips (Frantz and Mellinger 1990, Chau et al. 2005, CABI 2014)
Economic Importance
Thrips cause both direct and indirect injury to crops. Direct damage occurs when the thrips cause injury by feeding or oviposition (Fig. 9). Examples of direct injury caused by this species are scarring of pepper due to feeding (Funderburk et al. 2014a), and oviposition wounds in tomatoes (Funderburk et al. 2014b). Indirect damage refers primarily to the transmission of viruses by thrips. Examples of plant viruses transmitted by Frankliniella occidentalis include Tomato spotted wilt virus (TSWV) (Fig. 10), Tomato chlorotic spot virus (TSCV) (Fig. 11), Impatiens necrotic spot virus (INSV), and Groundnut ringspot virus (GRSV) (Fig. 12) (Fung et al. 2002, Frantz and Mellinger 2009, Hoddle et al. 2012, Frantz and Fasulo 2013, Webster et al. 2015).

Credit: Hugh Smith

Credit: Gary Vallad

Credit: Hugh Smith

Credit: Gary Vallad
Management
To sample for Frankliniella thrips or other flower-inhabiting thrips, select a set number of flowers, then either strike these flowers against a light-colored board and count the thrips while still in the field or place the flowers in a vial of ethanol and count the thrips later in the lab (Funderburk et al. 2014a, b). Blue sticky traps may also be used to sample thrips from the field. Blue traps were found to catch more Frankliniella occidentalis compared to yellow sticky traps, but yellow sticky traps have the advantage that they may also be used to monitor for leafminers, aphids, and whiteflies (Natwick et al. 2007, Frantz and Fasulo 2013, Muvea et al. 2014). It is possible to estimate of the population of thrips in the field with sticky traps, but trap catches have rarely been shown to reflect population size accurately or to be accurate predicators of damage. In addition, thrips caught on sticky cards may be difficult to identify to species.
In most circumstances only adults are identified, although keys exist to identify second instar larvae. Specimens are usually identified at 40X magnification. Specimens are slide-mounted using CMC-10 (temporary) or Canada balsam (permanent) mounting media. Museum-grade specimens are usually cleared with a solution of potassium hydroxide (Hoddle et al. 2012).
Cultural control techniques may be used to manage Frankliniella occidentalis. In northern Florida, UV-reflective mulch is used to reduce the number of thrips in tomato. UV-reflective mulch interferes with the host-finding behavior of thrips. (Stavisky et al. 2002, Demirozer 2014) (Fig. 13a and 13b). A reduction in nitrogen fertilization may result in a decrease in the number of Frankliniella occidentalis (Stavisky et al. 2002). Care must be taken when selecting an insecticide because thrips may build up resistance to an insecticide if it is applied too frequently. Resistance by western flower thrips to many groups of insecticides has been documented (IRAC 2014). Current information on insecticides available for thrips management in Florida can be found in the Vegetable Production Handbook for Florida.

Credit: Hugh Smith
Natural Enemies
Among the most important natural enemies of this species are minute pirate bugs, Orius spp. (Fig. 14). In pepper, suppression occurs at a ratio of approximately one Orius insidiosus to 180 thrips, and control occurs at a ratio of approximately one Orius insidiosus to 50 thrips (Funderburk 2009). Other thrips predators include the predatory mites Amblyseius swirskii and Neoseiulus cucumeris (Arthurs et al. 2009; Dogramaci et al. 2011).

Credit: Hugh Smith
Demirozer et al. (2012) reviewed effective, economical, and ecologically sound integrated pest management programs (IPM) for thrips in fruiting vegetables. The components included the following: define pest status (economic thresholds), increase biotic resistance (natural enemies and competition); integrate preventative and therapeutic tactics (scouting, ultraviolet [UV]-reflective mulch technologies, biological control, compatible insecticides, companion plants, and fertility); and vertically integrate the programs with other pests. These programs have been widely implemented in Florida, and they have significantly improved management of Frankliniella thrips and thrips-transmitted tospoviruses.
Selected References
Arthurs S, McKenzie CL, Chen J, Dogramaci, Brennan M, Houben K, Osborne L. 2009. Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biological Control 49: 91–96.
Brodsgaard HF. 1994. Insecticide resistance in European and African strains of western flower thrips (Thysanoptera: Thripidae) tested in a new residue-on-glass test. Journal of Economic Entomology 87: 1141–1146.
CABI. (2014). Invasive species compendium: Frankliniella occidentalis (Western Flower Thrips). Centre for Agricultural Bioscience International. https://www.cabi.org/isc/datasheet/24426. 20 December 2014.
Chambers RJ, Long S, Helyer NL. 2008. Effectiveness of Orius laevigatus (Hem.: Anthocoridae) for the control of Frankliniella occidentalis on cucumber and pepper in the UK. Biocontrol Science and Technology 3: 295–307.
Chau A, Heinz KM. 2006. Manipulating fertilization: a management tactic against Frankliniella occidentalis on potted chrysanthemum. Entomologia experimentalis et applicata 120: 201–209.
Childers CC, Beshear RJ. 1992. Thrips (Thysanoptera) species associated with developing citrus flowers in Florida and a key to adult terebrantian females. Journal of Entomological Science 27: 392–412.
Demirozer O, Tyler-Julian K, Funderburk J, Reitz S, Leppla N. 2012. Integrated pest management program for thrips and Tospoviruses in fruiting vegetables. Pest Management Science 68 (12): 1537–15458.
Dogramaci M, Arthurs SP, Chen J, McKenzie C, Irrizary F, Osborne L. 2011. Management of chilli thrips Scirtothrips dorsalis (Thysanoptera: Thripidae) on peppers by Amblyseius swirskii (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae). Biological Control 59: 340–347.
Frantz G, Fasulo TR. 2013. Thrips KnowledgeBase. Glades crop care. https://www.gladescropcare.com/thrips/pg1.html. 20 December 2014.
Frantz G, Mellinger HC. 1990. Flower thrips (Thysanoptera: Thripidae) collected from vegetables, ornamentals and associated weeds in south Florida. Proceedings of Florida State Horticultural Society 103: 134–137.
Frantz G, Mellinger HC. 2009. Shifts in western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), population abundance and crop damage. Florida Entomologist 92: 29–34
Funderburk J. 2009. Management of western flower thrips (Thysanoptera: Thripidae) in fruiting vegetables. Florida Entomologist 92: 1–6.
Funderburk J, Reitz SR, Stansly PA, Freeman JH, McAvoy E, Whidden AJ, Nuessly GS, Leppla NC. 2014a. Managing thrips in pepper and eggplant. University of Florida, Institute of Food and Agricultural Sciences, Electronic Data Information Source. ENY 658. https://edis.ifas.ufl.edu/publication/IN401. 29 August 2022.
Funderburk J, Adkins, S. Freeman J, Stansly P, Smith H, McAvoy G, Demirozer O, Snodgrass C. Paret M, Leppla N. 2014b. Managing thrips and tospoviruses in tomato. University of Florida, Institute of Food and Agricultural Sciences, Electronic Data Information Source. ENY 859. https://edis.ifas.ufl.edu/publication/IN895. 29 August 2022.
Fung SY, Kuiper I, Dijke-Hermans CM, van der Meijden E. 2002. Growth, damage and silvery damage in chrysanthemum caused by Frankliniella occidentalis is related to leaf food quality. pp. 191–196. In: Marullo R, Mound L. (eds), Thrips and tosposviruses: Proceedings of the 7th International Symposium on Thysanoptera, Australian National Insect Collection, Canberra, Australia. (20 December, 2014)
GBIF. 2014. Euthrips occidentalis Pergande, 1895. Global Biodiversity Information Facility. https://www.gbif.org/species/4423734. 20 December 2014.
Gonzalez D, Wilson LT. 1982. A food-web approach to economic thresholds: a sequence of pests/ predaceous arthropods on California cotton. Entomophaga 27: 31–43.
Hoddle MS, Mound LA, Paris DL. 2012. Thrips of California. CBIT Publishing, Queensland. http://keys.lucidcentral.org/keys/v3/thrips_of_california. (20 December 2014).
Hunter WB, Ullman. 1989. Analysis of mouthpart movements during feeding of Frankliniella occidentalis (Pergande) and F. schultzei Trybom (Thysanoptera: Thripidae). International Journal of Insect Morphology and Embryology 18: 161–171.
IRAC. 2014. Western flower thrips Frankliniella occidentalis. https://irac-online.org/pests/frankliniella-occidentalis/
Kindt F, Joosten NN, Peters D, Tjallingii WF. 2003. Characterization of the feeding behavior of western flower thrips in terms of electrical penetration graph (EPG) waveforms. Journal of Insect Physiology 49: 183–191.
Kirk WDJ, Terry LI. 2003. The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agricultural and Forest Entomology 5: 301–310.
Lewis T. 1991a. An introduction to the Thysanoptera: a survey of the group. In Parker BL, Skinner M, Lewis, T. (eds), Conference: Toward understanding Thysanoptera, Burlington, VT. 3–22. General Technical Reports NE-147, Randall, PA. U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. http://www.nrs.fs.fed.us/pubs/gtr/gtr_ne147/gtr_ne147_003.pdf
Lewis T. 1991b. Feeding, Flight and dispersal in thrips. In Parker BL, Skinner M, Lewis T. (eds), Conference: Toward understanding Thysanoptera, Burlington, VT. 63–70. General Technical Reports NE-147, Randall, PA: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. http://www.nrs.fs.fed.us/pubs/6365
Morse JG, Hoddle MS. 2006. Invasion biology of thrips. Annual Reviews of Entomology 51: 67–89.
Mound LA, Teulon DAJ. 1995. Thysanoptera as phytophagous opportunists, pp. 3–19 In Parker BL, Skinner M, Lewis T. (eds.), Thrips Biology and Management NATO ASI Series, Series A: Life Sciences Vol. 276. Plenum Press, New York.
Mound LA, Paris D, Fisher N. 2009. World Thysanoptera. CSIRO. http://anic.ento.csiro.au/thrips/identifying_thrips/Thripinae.htm. (20 December 2014.)
Muvea AM, Waignjo MM, Kutima HL, Osiemo Z, Nyasani JO, Subramanian S. 2014. Attraction of pest thrips (Thysanoptera: Thripidae) infesting French beans to coloured sticky traps with Lurem-TR and its utility for monitoring thrips populations. International Journal of Tropical Insect Science 34: 197–206.
Natwick ET, Byers, JA, Chu C, Lopez M, Henneberry TJ. 2007. Early detection and mass trapping of Frankliniella occidentalis and Thrips tabaci in vegetable crops. Southwestern Entomologist 32: 229–238.
Ramachandran S, Funderburk J, Stavisky J, Olson S. 2001. Population abundance and movement of Frankliniella species and Orius insidiosus in field pepper. Agricultural and Forest Entomology 3:1–10.
Reitz SR. 2009. Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): the making of a pest. Florida Entomologist. 92: 7–13.
Reitz SR, Yu-lin G, Zhong-ren L. 2011. Thrips: pests of concern to China and the United States. Agricultural Sciences in China 10: 867–892.
Salguero-Navas VE, Funderburk JE, Beshear RJ, Olson SM, Mack TP. 1991. Seasonal patterns of Frankliniella spp. (Thysanoptera: Thripidae) in tomato flowers. Journal of Economic Entomology 84: 1818–1822.
Shipp JL, Wang K. 2003. Evaluation of Amblyseius cucumeris (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae) for control of Frankliniella occidentalis (Thysanoptera: Thripidae) on greenhouse tomatoes. Biological Control 28: 271–281.
Stavisky J, Funderburk J, Broadbeck BV, Olson SM, Andersen PC. 2002. Population dynamics of Frankliniella spp. and Tomato spotted wilt incidence as influenced by cultural management tactics in tomato. Journal of Economic Entomology 95: 1216–1221.
Terry LI, Gardener, D. 1990. Male mating swarms in Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Journal of Insect Behaviour 3: 133–141.
Webster C, Frantz G, Reitz S, Funderburk J, Mellinger HC, McAvoy E, Turecheck WW, Marshall SH, Tantiwanich Y, McGrath M, Daughtrey M, Adkins S. 2015. Emergence of groundnut ringspot virus and tomato chlorotic spot virus in vegetables in Florida and the southeastern United States. Phytopathology 105: 388–398.
Zhang ZJ, Wu QJ, Li XF, Zhang YJ, Xu BY, Zhu GR. 2007. Life history of the western flower thrips, Frankliniella occidentalis (Thysan., Thripidae), on five different vegetable leaves. Journal of Applied Entomology 1361: 347–354.