Introduction
The purpose of this guide is to provide Florida cotton growers a selected set of options for integrated pest management (IPM) of arthropods (insects and mites) in their fields. It serves as a reference for cultural, mechanical, biological, and chemical control of arthropods. Included are links to additional UF/IFAS EDIS articles, as well as external sources of information on arthropod management. The guide also contains a "Searchable Table of Registered Insecticides, Herbicides, and Fungicides for Florida Cotton."
Arthropods impact cotton crop yields through direct herbivory. The feeding damage to roots, leaves, stems, and fruit can significantly reduce overall plant health and productivity. Damage can be caused by various life stages of these pests that could be located in different places on or within the plant or field, frequently making them difficult to find and identify and subsequently costly to manage. Feeding damage often can be used to locate cotton pests if growers understand the basic life cycle and biology of their arthropod pests.
Major Categories of Arthropod Pests on Cotton
While many different species of arthropods affect cotton, the primary pests can be categorized into the following groups: thrips, lepidopteran (moth) larvae, the boll weevil, plant bugs, aphids, mites, and white flies (see identification section).
Several species of flower thrips occur on cotton seedlings, including tobacco thrips, western flower thrips, and another species usually referred to as the eastern flower thrips, Frankliniella tritici. Eggs deposited directly into the plant tissue hatch within days, and the seedlings are inhabited by both adults and larvae. These thrips stages can cause significant foliar injury, and some also vector pathogens that cause cotton diseases.
Cotton can be damaged by lepidopteran pests from seedling emergence to harvest. Cutworms infest seedlings, and soybean loopers feed on leaves from mid to late season. Cotton bollworms and tobacco budworms infest terminal areas, developing squares, and bolls. Beet and fall armyworms develop from egg masses deposited on leaves, and, after hatching, disperse to other parts of the plant, including squares, flowers, and bolls.
The boll weevil deposits its eggs in developing cotton squares, and the larvae consume the contents. Historically, this species was a serious pest of cotton in Florida, but success of the area-wide eradication program has nearly eliminated it from the state. The USDA Animal and Plant Health Inspection Service (APHIS) released an eradication program factsheet in 2007 (https://www.fdacs.gov/content/download/10151/file/faq_boll_weevil_07.pdf).
True bug pests in cotton include species of plant bugs, plant hoppers, and stinkbugs. These insects pierce the flowers and buds with their mouthparts and suck the contents. Plant bug injury can affect square retention, and stink bugs are directly injurious to bolls.
Although not yet a significant problem for Florida cotton growers, the cotton seed bug, Oxycarenus hyalinipennis, is being monitored for its potential impact. The Florida Department of Agriculture and Consumer Services, Division of Plant Industry (FDACS, DPI) released a Pest Alert describing O. hyalinipennis (https://www.fdacs.gov/content/download/68511/file/Pest%20Alert%20-%20Oxycarenus%20hyalinipennis,%20Cotton%20Seed%20Bug.pdf).
Identification
It can be difficult to locate and identify arthropods in a cotton crop using field guides. However, identification is facilitated by using publications on arthropods commonly found on cotton available online through UF/IFAS EDIS (https://edis.ifas.ufl.edu), IPM Florida (https://ipm.ifas.ufl.edu), and publications from other institutions:
- Cotton insect identification—https://extension.msstate.edu/sites/default/files/publications/publications/p1640.pdf
- Tobacco thrips—https://ipm.ifas.ufl.edu/pdfs/Tobacco_thrips.pdf
- Tobacco budworm—https://edis.ifas.ufl.edu/publication/IN376
- Cotton bollworm—http://www.ars.usda.gov/Research/docs.htm?docid=7599
- (True) armyworm—https://edis.ifas.ufl.edu/publication/IN702
- Beet armyworm—https://edis.ifas.ufl.edu/publication/IN262
- Fall armyworm—https://edis.ifas.ufl.edu/publication/IN255
- Cutworms—https://extension.tennessee.edu/publications/Documents/W032.pdf
- Loopers—https://extension.tennessee.edu/publications/Documents/W034.pdf
- Boll weevil—http://ipm.ncsu.edu/AG271/cotton/boll_weevil.html
- Plant bugs and stink bugs—http://www.clemson.edu/psapublishing/Pages/Entom/EB158.pdf
- Melon (cotton) aphid—https://edis.ifas.ufl.edu/publication/IN330 and https://edis.ifas.ufl.edu/publication/IN1055
- Broad mite—https://edis.ifas.ufl.edu/pdffiles/IN/IN105300.pdf
- Two-spotted spider mite—https://edis.ifas.ufl.edu/publication/IN1059
- Silverleaf whitefly—https://edis.ifas.ufl.edu/publication/IN1057
Scouting and Damage Thresholds
Cotton fields require frequent scouting from emergence of the crop to harvest because damaging populations of arthropods can develop quickly. Alternative cultural or mechanical practices and pesticides are used to prevent a pest population from reaching a damage threshold. This IPM approach, whether growing Bt or non-Bt cotton, is the best way to manage arthropod pests. The unnecessary use of pesticides can incur costs to the grower, exacerbate pest problems, and damage the environment. More specifically, application of broad-spectrum pesticides can reduce natural enemy populations, be long-lasting, and result in target pest resurgence, secondary non-target pest outbreaks, and pesticide resistance in both target and non-target pests.
Thrips have rasping-sucking mouthparts they use to ingest cotton plant cell contents. Mouthparts are asymmetrical and scrape the surface of the leaves, damaging the plants. Thrips are usually present on some cotton seedlings and tend to occur randomly throughout a field. Moderate injury is not damaging if seedlings are sufficiently vigorous, so it is important to maintain seedling vigor. Severe tobacco and other thrips injury, especially in combination with other stressors from drought and some pesticides, can delay maturity. Thrips can either be managed preventively using insecticides at planting or therapeutically if populations reach the damage threshold of 2–3 thrips per plant with immatures present. Treatment of vegetative cotton after four leaves are present is not justified. The decision to use at-plant treatment for thrips is problematic. Because adults overwinter, fields planted before May 10 using conventional tillage are at high risk; whereas, fields planted after May 10 using no-tillage are at low risk. Treatment with neonicotinoid insecticides harms important predator populations. The harmful effects may last for months, thereby contributing to pest population resurgence. There are reports from several southern states that thrips have developed resistance to neonicotinoid seed treatments.
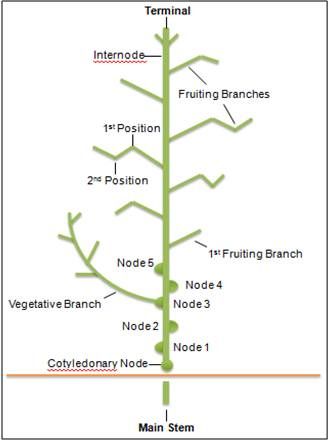
Larval tobacco budworms and cotton bollworms feed on cotton leaves and other parts of the plant. When scouting for these pests on non-Bt cotton, randomly select plants and examine them for the presence of eggs and small larvae. Concentrate on the terminal areas and upper 8 to 12 inches of the plant (Figure 1). Eggs are deposited in the terminal areas or on the upper surface of newly expanded leaves, and also on the outer bracts. Larvae can be found in terminal buds and inside developing squares. Scout for cotton bollworms and tobacco budworms by determining the number of small larvae (1/4 inch in length or less) per 100 plants. Some states recommend a first insecticide application when there are 8 small larvae per 100 plants. Thereafter, 5 larger larvae per 100 plants is the threshold. Currently available Bt cotton varieties provide good to excellent control of tobacco budworms and good control of bollworms and most other caterpillars. However, high populations of bollworms may require treatment in some situations. In Bt cotton, scout for larvae ¼ inch or greater in length in blooms, squares, and bolls.
Credit: Adapted from Silvertooth (2001).
Armyworms place egg clusters on the undersides of leaves where the newly hatched larvae feed before dispersing. The larvae are easiest to control with insecticides when they are small, especially before they migrate to flowers and bracts, where they are protected from insecticide spray. Scout for beet and fall armyworms by counting the number of "hits" (newly hatched egg clusters) per length of row. The damage threshold for beet armyworms is 10 "hits" per 300 feet of row, 10% of blooms infested, or 10% of squares damaged. For fall armyworms, an economic threshold of 15 larvae per 100 plants is frequently used.
Cutworms typically aggregate on cotton seedlings, resulting in discontinuous stem damage within a field. Therefore, an entire field must be assessed for damage. Look for injury due to an active infestation, and determine average plant stand density in each area of the field. Apply insecticides to areas of a field where active infestations could reduce the plant density below acceptable levels.
Loopers are occasional pests that cause cotton plant defoliation, especially during the mid and late season. Scouting considerations include the number of loopers present, time to crop maturity, and potential for excessive premature defoliation. Percent defoliation can be estimated when assessing cabbage and soybean looper damage. An insecticide application is warranted if pest populations cause damaging levels of defoliation when immature bolls are present. A groundcloth can be used to determine the number of small and large larvae per row foot.
Boll weevil larvae and adults damage cotton plants by feeding on squares or boll tissue. In severe infestations, adult females lay an egg on nearly every square that is suitable to produce a larva. Squares and small bolls fed on by adults usually drop before developing fully. Stunting is common, and fruit do not set properly, resulting in substantial yield losses. Check for boll weevils during spring and fall. Some states recommend spring insecticide applications for overwintered weevils when 5th node growth is reached. These "pinhead sprays" are generally effective against the first generation of weevils until August and should not be applied more than twice during the season, particularly not if the cotton squares past mid-June (Head and Williams 2014). If pheromone traps are used at a density of 1/20 acres, consider "pinhead" square insecticide applications if an average of one weevil per trap is collected four weeks before squaring. When traps are not in use, a threshold of 25 or more weevils per acre warrants chemical control. Once the cotton squares are 1/3 grown, damage levels above 10% should be treated every 4th day until control is established. Lastly, if weevils are problematic mid-to-late season, or if the crop remains green during the fall, stalk destruction is recommended along with adding boll weevil-specific insecticides to defoliants.
Plant and stink bugs damage cotton squares and bolls by feeding. Scout pre-square cotton visually in several areas of a field, and estimate the number of plant bugs per 6 feet of row. After squaring begins, adult plant bugs can best be sampled using a 15-inch-diameter sweep net. Take several 25-sweep samples per field. Space the sweeps widely apart, and move quickly down the row while sweeping. The plant bug nymphs are best sampled by placing a 3-ft.-wide by 6-ft.-long groundcloth between the rows and beating the plants on both sides of the row. Determine the number per sample (6 feet of row). Flowering and boll development are monitored to assess the impacts of plant bugs. Buds or squares begin to appear in about 4 to 5 weeks after planting on fruiting branches produced at each successive main stem node, beginning with the sixth or seventh node. Monitor the percentage of square retention by counting the number of missing squares from 100 first or second position square sites (Figure 1), as indicated by the presence of an abscission scar. From plants with more than 5 fruiting branches, limit counts to potential fruiting positions on the 5 uppermost fruiting branches. An insecticide application is justified when numerous bugs are present and the plants are retaining less than 80% of pinhead squares. An insecticide application may also be justified during the first 2 weeks of squaring when groundcloth samples reveal 1 plant bug per 6 feet of row or sweep net samples detect 8 bugs per 100 sweeps. From the third week of squaring through bloom, treat when groundcloth samples yield 3 bugs per 6 feet of row or sweep net samples detect 15 bugs per 100 sweeps. For stink bugs, an average of 1 per 6 feet of row justifies treatment. Thresholds based on boll injury also are used with an injury threshold of 10–15% during weeks 3–5 of bloom, 20% during weeks 2 and 6, and 30% beginning week 7.
Aphids damage cotton plants by direct feeding with their piercing-sucking mouthparts. All life stages cause foliar damage, which can appear as chlorotic spots. Severe infestations on plant terminals and young leaves can result in distorted growth, often including stunted plants and downward-cupped leaves. The honeydew aphids secrete also damages cotton plants. It collects on the surface of plant tissues and induces the growth of sooty mold. Sooty mold can reduce photosynthesis and overall cotton productivity. These aphid pests are controlled by natural enemies that occur in cotton fields, including a fungal pathogen. Epidemics of this fungal pathogen occur only after aphid populations reach high levels. Insecticide applications for cotton and other aphids may be justified when the leaves of seedling cotton are severely curled, or when heavy infestations cause honeydew-coated plants on older cotton.
Mites damage cotton by piercing epidermal cells on the undersides of cotton leaves. Overall water loss, chlorotic spots, defoliation, and reduction in boll development can result. Examine the 5th leaf below the terminal for the presence of two-spotted spider mites, and determine the percentage of plants infested. An insecticide application is justified when leaves begin to redden and mite populations are spreading in the field. Consider spot treating areas of the field where populations are aggregated.
Whiteflies, like other true bugs, damage cotton through direct feeding with piercing-sucking mouthparts. Dense infestations of whiteflies can also cause honeydew buildup on leaf tissues that reduces growth of the cotton plant. Cotton leaf curl virus is also vectored by whiteflies. Scout for silverleaf and other whiteflies by determining the percentage of terminals infested. Examine the undersides of the upper leaves of the plant. The threshold for banded winged whiteflies is 50% of the terminals infested for rapidly growing cotton or when honeydew is found on the foliage or lint. Silverleaf whiteflies are difficult to control with insecticides, so conservation of natural enemies is important.
Cultural Control
Fertilization and Irrigation
Certain cultural methods, such as excessive nitrogen use and irrigation, can delay maturity and increase damage from late-season insects. Therefore, it is important to properly manage soil fertility and moisture levels both during and between growing seasons.
Crop Density
High crop density can significantly affect overall cotton quality and quantity by increasing the plant's susceptibility to arthropod pests. Dense stands can stress the crop via intraspecific competition among individual cotton plants. The plants compete for optimum growing conditions involving space, water, and nutrients. An excessively dense stand results in delayed fruit initiation and maturity, thus increasing exposure to late-season insects.
Cultivar Choices
Many of the more serious cotton pests, including tobacco budworms, cotton bollworms, armyworms, stinkbugs, and loopers, are most damaging late in the season. Therefore, the selection of early-maturing cotton varieties is an effective method of escaping highly damaging populations of these arthropod pests.
Mechanical Control
Mechanical arthropod control via tillage can prove effective in reducing populations of soil-dwelling larvae and pupae. However, it is important to gauge the frequency and depth of tillage practices because they can increase erosion and reduce overall soil health. Additionally, cotton roots can be damaged if in-season tillage is conducted too late. The type and cost of the equipment needed for tillage depends on the size of the cotton crop.
Fall tillage at an appropriate depth destroys some overwintering pupae of tobacco budworms and cotton bollworms. Spring tillage at least three weeks before planting minimizes the risk of cutworm problems during the growing season. Early spring tillage that occurs after planting also will eliminate some overwintering pupae of tobacco budworms and cotton bollworms.
Biological Control
Biological control is accomplished by importing and releasing natural enemies from outside the United States, producing them commercially for augmentative releases, and conserving those that exist naturally. Cotton fields are inhabited by a complex of arthropod natural enemies that can build up and significantly reduce pest populations. Predators include bigeyed bugs, minute pirate bugs, damsel bugs, ladybugs, lacewings, predatory stink bugs, and ground beetles. Each pest also has a complex of egg and or larval parasitoids. The environment can be modified to support these predators and parasitoids, and, if not killed by pesticides, they often can maintain pest insect populations below damage thresholds.
Many beneficial arthropods are available for purchase from commercial producers or suppliers for augmentative release, e.g., predatory lacewing larvae and ladybugs that control aphids, whiteflies, and additional pests (LeBeck and Leppla 2015). Also available are parasitoid wasps that control lepidopteran larvae. Microorganisms, such as bacteria or fungi that are pathogenic to specific arthropods, are formulated into biopesticides or in some cases incorporated into the cotton plant. For example, genetically engineered Bt cotton contains toxin-producing genes from the bacterium Bacillus thuringiensis. These toxins kill larvae that attempt to feed on the cotton plant.
Chemical Control
Although Extension agents and industry professionals generally recommend that cotton growers adopt IPM of arthropod pests of cotton, chemical control alone remains the commonly accepted and oftentimes cost-effective approach. Therefore, growers must routinely monitor their maturing crops for pests and terminate pesticide use when the cotton is reasonably safe from insect damage. Over-application of chemical pesticides can induce resistance development among pest species and lead to increased crop damage.
Bt Cotton Effectiveness
Bt cotton is commercially available in three varieties: Bollgard II, WideStrike, and WideStrike III. Both Bollgard II and Widestrike are 2-gene Bt cotton varieties containing Cry1Ac toxins. Bollgard II also contains Cry2Ab toxins, and WideStrike contains Cry1F toxins. WideStrike III is a three-gene Bt cotton, containing not only Cry1Ac and Cry1F, but also Vip3A. While Bt cottons generally provide excellent control of most lepidopteran larvae, they are not immune to damage caused by other arthropods, especially true bug pests such as plant and stink bugs. Thorough scouting is imperative throughout the growing season, and supplemental insecticide applications may be necessary to control certain pests.
Bt Cotton Resistance Management
Arthropod resistance to Bt cotton technologies is becoming a more common concern for growers. With season-long exposure of lepidopteran larvae, resistance to Bt cotton may develop quickly if resistance management tactics are not employed. The "refuge approach" involves maintaining a population of susceptible moths to mate with potentially resistant moths derived from larvae that fed on the Bt cotton. Refuges for pest Lepidoptera are provided in non-Bt cotton fields planted near the Bt cotton. Growers should implement the required resistance management practices according to use agreements provided by their supplier of transgenic seed technology. The US Environmental Protection Agency provides useful resistance management information: https://www.epa.gov/regulation-biotechnology-under-tsca-and-fifra/insect-resistance-management-bt-plant-incorporated
Pesticide Table
The table below compiled from the National Pesticide Informational Retrieval System (NPIRS) lists the major arthropod pests of cotton in Florida, the active ingredients and example products registered for controlling them, and the Insecticide Resistance Action Committee (IRAC) classification for use in rotating active ingredients to prevent resistance in target pests. The last column indicates special precautions and recommendations for maximizing arthropod control. To use the table efficiently, a grower first should identify the problematic arthropod species in the "Arthropods Controlled" column. The prevailing active ingredients to control the arthropod are then listed in alphabetical order in the second column along with the trade name of one commonly used product. In some cases, a blend of active of ingredients may prove to be more effective either pesticide alone.
References
Allen, C., J. Bibb, D. Cook, D. Dodds, J. Gore, T. Irby, E. Larson, B. Layton, J. MacGown, S. Meyers, and F. Musser. 2016. Insect Control Guide for Agronomic Crops. Mississippi State University Extension Publication 2471. https://extension.msstate.edu/sites/default/files/publications/publications/p2471_0.pdf
Green, J. K., C. S. Bundy, P. M. Roberts, and B. R. Leonard. 2006. Identification and Management of Common Boll-Feeding Bugs in Cotton. Clemson University. EB 158. Accessed 12 August 2022. https://www.cottoninc.com/wp-content/uploads/2015/12/Boll-Feeding-Cotton-Bugs-Mgmt.pdf
Huseth, A. S., T. M. Chappell, K. Langdon, S. C. Morsello, S. Martin, J. K. Greene, A. Herbert, A. L. Jacobson, F. Reay-Jones, T. Reed, D. D. Reisig, P. M. Roberts, R. Smith, and G. G. Kennedy. 2016. "Frankliniella fusca resistance to neonicotinoid insecticides: an emerging challenge for cotton pest management in the eastern United States." Pest Manag. Sci. 10:1002–1013.
LeBeck, L. M., and N. C. Leppla. 2015. Guidelines for Purchasing and Using Commercial Natural Enemies and Biopesticides in North America. Gainesville: University of Florida Institute of Food and Agricultural Sciences. IPM-146. https://edis.ifas.ufl.edu/publication/IN849
Mossler, M. A. Florida Crop Pest management Profile: Cotton. Gainesville: University of Florida Institute of Food and Agricultural Sciences. PI-220. https://edis.ifas.ufl.edu/pi220
Naranjo, S. E., and P. C. Ellsworth. 2009. "The contribution of conservation biological control to integrated control of Bemisia tabaci in cotton." Biol. Control. 51:458–470. https://www.sciencedirect.com/science/article/abs/pii/S104996440900214X
Silvertooth, J. C. 2001. "Estimating Fruit Retention." University of Arizona Cooperative Extension, Publication az1208. https://ag.arizona.edu/crops/cotton/cropmgt/fruit_retention.html
Sorenson, C. E. 1995. "The Boll Weevil in Missouri: History, Biology and Management." MU Delta Center. University Extension, University of Missouri-Columbia. Agricultural MU Guide. Accessed 12 August 2022 https://extension.missouri.edu/publications/g4255
Steinkraus, D., J. Zawislak, G. Lorenz, B. Layton, and R. Leonard. Spider Mites on Cotton in the Midsouth. University of Arkansas Division of Agriculture. http://www.cottoninc.com/fiber/AgriculturalDisciplines/Entomology/Spider-Mites/Spider-Mites-Cotton/Cotton-Spider-Mites.pdf
Whitaker, J., S. Culpepper, G. Harris, B. Kemerait, C. Perry, W. Porter, P. Roberts, D. Shurley, and A. Smith. 2016. Georgia Cotton Production Guide. Cooperative Extension, University of Georgia, College of Agricultural and Environmental Sciences. http://www.ugacotton.com/vault/file/2016-UGA-Cotton-Production-Guide.pdf
Table of Registered Pesticides for Florida Cotton
Access the "2016 Pesticide List for Florida Cotton" here: http://goo.gl/3DJ2pz.
This table serves as a comprehensive list of pesticides registered in Florida for use on cotton. The list was generated in 2016 from the National Pesticide Information Retrieval System database.
To filter the list of pesticides and search for specific pests, products, active ingredients, or IRAC resistance codes, you must first click on the filter icon located in the gray toolbar (indicated below by the red box). You may create multiple temporary filter views to compare lists that have different sorting options or filters. Each "temporary filter view" can be customized according to your desired results.
Next, click "Create a new temporary filter view." Your new spreadsheet should look like this. A dark grey bar that says "Temporary filter 1" should be located at the top of the spreadsheet.
Click "Dismiss" for the popup window regarding the temporary filter view if it appears in the top left to close it.
Now you can begin to filter and search through the comprehensive list for target pests and registered pesticides. Here are filtering instructions with some examples based on desired results:
View only chemicals registered for a specific target pest
The majority of target pests listed in this table are identified by a single common name. However, there are some exceptions that do not have a common name or that have multiple common names. Broader pest categories, such as aphids or thrips, can be selected within the comprehensive target pest lists. It is necessary to consult the pesticide label when considering its use to control a specific pest.
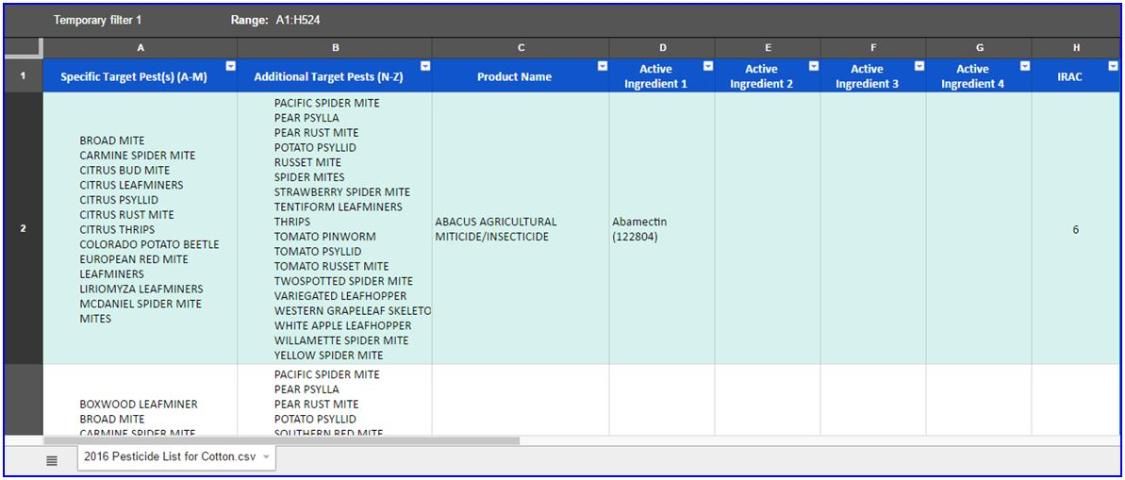
To begin, first select the appropriate column for your pest. The cells in column A contain the common names of "Specific Target Pest(s) A–M" and column B "Additional Target Pests (N–Z)."
Click on the drop-down arrow at the top right corner of the first cell in the appropriate column.
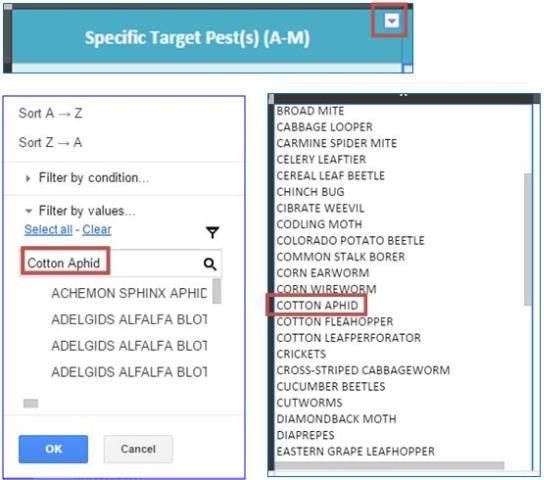
Enter the common name of the target pest, pathogen, or weed you wish to filter for.
For example: "Cotton Aphid" in column A.
- Then, click "Select All" and "OK." Remember to search for terms that fall within the alphabetical range of column A or B.
- The table now is repopulated with only the insecticides that have "Cotton Aphid" listed as a target pest in column A. This can be verified by selecting an individual cell in column A and scrolling down to locate the pest.
All of the pests, pathogens, or weeds now shown in Columns A and B are controlled by the pesticide product in Column C with active ingredients in Columns D and E.
Helpful Hints:
IMPORTANT: Remember to clear any filters before starting a new search. You can determine which columns still have a filter in use by locating the green filter icons in the top right corner of the header cells.
To undo a filter and return the spreadsheet list to its original composition, locate the green filter icon in the top right corner of the column you searched.
Click on the green filter icon for the drop down window to appear.
Click "Clear," then "Select All," and lastly "OK."
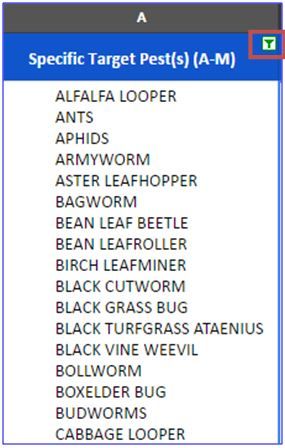
- If a cell looks empty, click on it and scroll down to view its contents.
- To search for a more general category of pests, such as "Thrips," enter the category as your keyword in the appropriate column (A for A–M or B for N–Z).
- The list will contain only pesticides that have been registered for that broad pest category, and not those that are pest-specific.
- Additionally, pesticides that are registered for a broad pest category might not be equally effective against all members. Follow the label and contact a local county Extension office for additional information.
View only chemicals with a specific primary active ingredient
Pesticides should be used efficiently and properly applied to ensure maximum effectiveness and minimum non-target effects on other organisms and the surrounding environment. The active ingredient (or in some cases, multiple ingredients) is the compound responsible for initiating control of target pests. These compounds may alter the biochemistry of the host plant to make it less attractive or even harmful to the pests. Other active ingredients target the pest's physiology directly.
To search for pesticides containing a specific primary active ingredient, click on the drop down arrow at the top right corner of the header in column D, "Active Ingredient 1."

Click "Clear" to begin a new active ingredient search. You can use the scroll bar on the right side of the window to scroll through the list of primary active ingredients.
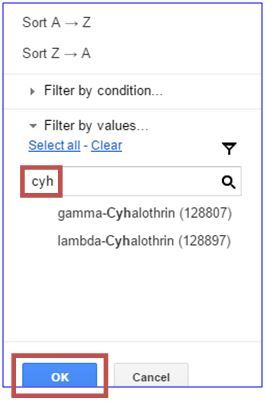
Alternatively, if you know the exact name of the ingredient, you can enter it in the search bar.
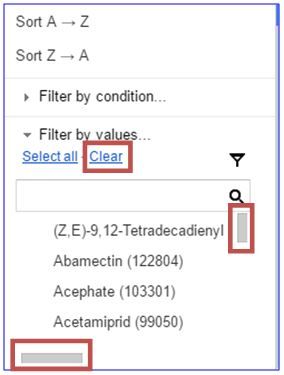
Searches are more difficult when active ingredients are listed in blends. It may help to enter the first few letters of the active ingredient's name and use the scroll bars on the right and bottom of the drop-down window to view the options. When you select an active ingredient, a check mark will appear to the left of it. Alternatively, if you know the exact name of the ingredient, you can enter it in the search bar.
Click "OK" to generate a list of pesticides with that active ingredient. To create a list of pesticides containing more than one primary active ingredient, clear the search and repeat the procedure. You can select as many active ingredients as are available for pesticides registered to manage the target pest. The products listed in the table now contain the active ingredient you filtered for and all the pests, pathogens, or weeds those products control are listed in Columns A and B.
View only chemicals with a designated IRAC group
The IRAC classification of pesticides into groups and sub-groups is based on the mode of action of the active ingredients. The primary purpose of this scheme is to minimize the development of pesticide resistance by the target pests. Pesticides from different groups are alternated to assure that pests do not become resistant to any of the active ingredients.
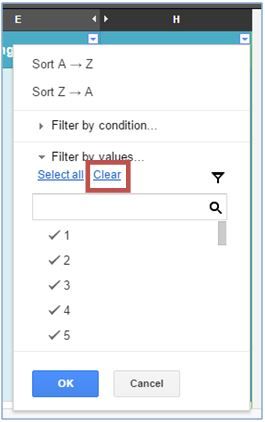
To search for chemicals containing a specific IRAC group click the drop down arrow in the top right corner of cell H1.

Click "Clear" to begin a new IRAC group search. The checkmarks beside the listed numbers will disappear. You can directly scroll through the list of groups by using the scroll bar on the right side of the window. If you know the exact group, you can enter it into the search bar and a check mark will appear to the left after it is selected. Click "OK" to generate a list of pesticides with that IRAC group. To create a list of pesticides for another group, clear the search and repeat the procedure. You can select as many codes as are available for pesticides registered to manage the target pest.
All of the IRAC groups are displayed in the H column. If a pesticide has multiple active ingredients, the group of the primary active ingredient is listed first. In some cases, pesticides may be registered for control of mites and other arthropods, or weeds, these products may have a FRAC or HRAC code associated with them. Only IRAC groups are included in this table. Those products with an "UN" have been classified as having an "unknown mode of action" by IRAC and appear with this specific group code in the IRAC classification scheme. Those products with an "N/A" do not yet have a mode of action classified by IRAC and therefore do not appear in any of the IRAC groups. The following websites contain additional information on the classification schemes:
Insecticide Resistance Action Committee—https://irac-online.org/modes-of-action/
Herbicide Resistance Action Committee—https://hracglobal.com/tools/hrac-mode-of-action-classification-2022-map
Fungicide Resistance Action Committee—https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2022--final.pdf