The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The broad-tipped conehead katydid, Neoconocephalus triops (Linnaeus, 1758), is a coneheaded katydid in the subfamily Conocephalinae. Within this subfamily, four genera consisting of 22 species total, exist in America (SINA 2014a). Like all other coneheads, they possess a cone (called a fastigium), a projection (sometimes sharply pointed, depending on the species) from the head that stretches beyond the basal antennal segments (SINA 2014c, Capinera et al. 2004). However, unlike other species in Conocephalinae, the broad-tipped coneheads bear fastigia that are wider than they are long (Figure 2).
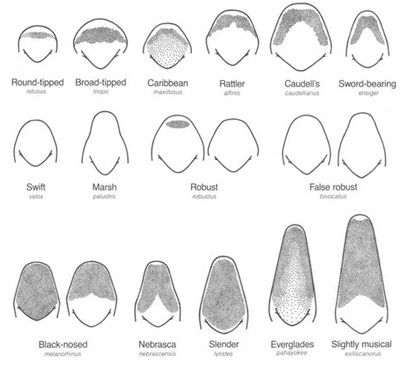
Credit: T. J. Walker, UF/IFAS (Accessed at http://entnemdept.ufl.edu/walker/buzz/g185a.htm on September 24, 2015)
Adult broad-tipped coneheads display either brown or green coloration, depending on the sex and season. Little is known regarding the adaptive significance or the genetic and environmental determinants of this katydid's color forms. However, the adaptive significance does correlate to predator protection during the daytime, and research supports that photoperiods affect male coloration (Whitesell and Walker 1978). Additionally, these coneheads make distinct calls during the summer and winter/early spring in North America.
Synonymy
Neoconocephalus triops (Linnaeus, 1758)
Gryllus triops Linnaeus, 1758
Conocephalus triops (Linnaeus) Scudder, 1869
Neoconocephalus macropterus Redtenbacher, 1891
Neoconocephalus triops (Linnaeus) Rehn & Hebard, 1915
Obtained from: Neoconocephalus triops, Encyclopedia of Life.
(For a continually updated list, visit the Orthoptera Species File.)
Distribution
Neoconocephalus triops has the most extensive range of all the species within the genus Neoconocephalus. They are mostly found near tropical areas (T.J. Walker, personal communication). Coneheads have been reported in the Caribbean on the islands of the Greater and Lesser Antilles and in Trinidad; and in Central and South America in Guyana, Panama, Peru, and on the Galapagos Islands of Ecuador (Walker and Greenfield 1983). This species also occurs throughout the southern United States, from Florida up to New Jersey, and extends westward to southern California. In Arizona, California, Delaware, Kentucky, New Mexico, and Tennessee only spring-singing katydids have been noted. Both spring- and summer-singing Neoconocephalus triops inhabit all parts of the state of Florida, including the Keys (Figure 2).
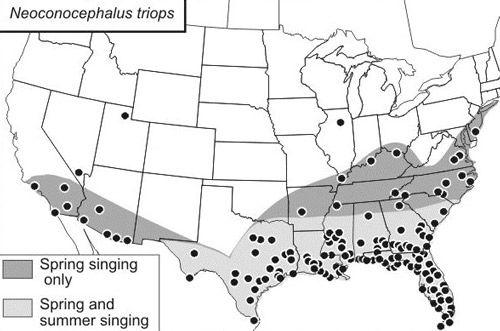
Credit: Computer-generated distribution map produced in 2003 from records in the GrylTett database.
Description
Coneheaded katydids have oversized jaws and a relatively large body, with adult females (except for Belocephalus spp.) generally much larger than males. Their bodies are covered by long, narrow, leathery forewings (SINA 2014a, Capinera et al. 2004). Both adult males and females are strong fliers and tend to be attracted to lights (SINA 2014b). When disturbed, adult coneheads will fly off or dive down into the ground, burying their head to make their body appear as grass (Figures 3 and 4) (Capinera et al. 2004).

Credit: T. J. Walker, UF/IFAS

Credit: T. J. Walker, UF/IFAS
Neoconocephalus, like most adult male katydids, produce a calling song by rubbing their wings together. In order to call mates in late spring, males perch from treetops at night; in early spring and summer, males call from understory and/or weedy areas (T.J. Walker, personal communication). The different seasonal generations of the broad-tipped coneheads were once thought to be two separate species because of the noticeable differences in the mating calls; the summer generation produces calls (typically in April; July to August in two-generation areas) structured in verses, while the winter/early spring generation produces continuous calls (typically in February to May) (SINA 2014b). However, because tachinid flies can locate males from their calls, the flies parasitize them and kill off the majority, so calls might become less frequent after February (T.J. Walker, personal communication). Additionally, under equal temperatures, the average wingstroke rate for summer calls is over 20% higher than the winter/early spring calls (Beckers and Schul 2008). Both calls are nearly equally attractive to adult females. It has been suggested that the calls differ because summer verses allow calling males a pause to detect other competing males, while lower population densities in the winter/early spring generation make this pause unnecessary (Beckers and Schul 2008).
Life Cycle
Eggs
The eggs are light green in color and are long and oval-shaped with pointed ends (Figure 5). Coneheaded katydids oviposit in grasses in between stems and sheaths of roots or leaves (SINA 2014a). In captivity, oviposition in the soil has been recorded, and the captive-bred hatchlings emerge after about eight weeks (Whitesell 1974). All other Neoconocephalus spp. overwinter as eggs except for the broad-tipped conehead, which overwinters during the adult stage (Capinera et al. 2004).

Credit: Oliver M. Beckers, University of Missouri–Columbia
Young
Broad-tipped conehead katydid nymphs are light green, resembling the adults with long, slender bodies, but lack wings and sexual characteristics. Typically these katydids go through seven instars (SINA 2014b). Early instars possess a distinct white line running from the fastigium to the tip of the abdomen (Figure 6). Although these katydids can display brown coloration as adults, the brown coloration has not been observed at egg emergence. Rather, they change from green to brown during late-instar molts, usually the last molt (SINA 2014a). Juveniles of the Neoconocephalus spp. are found among grasses, feeding on the grass flowers and developing seeds (SINA 2014c). Nymphs reach adulthood after about seven weeks (Whitesell 1974).

Credit: Oliver M. Beckers, University of Missouri–Columbia
Adults
Adult females tend to be larger than adult males, measuring 51 to 67 mm and 43 to 60 mm, respectively (Capinera et al. 2004). In adult females, the ovipositor is about as long as the hind femur (SINA 2014b).
The broad-tipped conehead is the only species within the suborder Ensifera in which development influences the mating call (Walker and Greenfield 1983). The mating call of this species can be heard in early spring, occurring at least a month before any other Neoconocephalus sp. due to its overwintering stage (Capinera et al. 2004, T. J. Walker, personal communication).
Overwintering adults inhabit thickets and woody areas (SINA 2014b). In early spring, the overwintered individuals become reproductively active. The offspring of one generation generally produce the next generation, so the winter/early spring and summer generations make up a single gene pool (Beckers and Schul 2008). The progeny of the spring generation become adults in mid-summer or early fall, but will not become reproductively active until the next spring (Capinera et al. 2004). In the northern United States, the broad-tipped conehead exhibits a univoltine life cycle, producing one generation per year. In Florida, this species has been observed as univoltine and bivoltine (produces two generations per year) (Whitesell 1974).
About 95% of males in the overwintering population are brown (Figure 7), while about 30% of overwintering females are brown. Only about 20% of the summer males and females display brown coloration and the remaining adults are green (Figure 8) (Capinera et al. 2004).

Credit: T. J. Walker, UF/IFAS

Credit: Shari Linn, UF/IFAS
Host Plants
The typically green broad-tipped conehead juveniles and feeding adults frequent grasses such as bahiagrass (Paspalum notatum) and Vaseygrass (Paspalum urvillei) to eat grass seed (Whitesell 1974).
The broad-tipped conehead has been classified as potentially harmful to Venezuelan sugar cane, but is not currently considered economically injurious (Guagliumi 1960).
This katydid has not been reported to damage economically important crops in Florida.
Management
Generally, katydids do not reach high population levels that would require management. If they do become a problem, both the nymphs and adults contribute to the damage. Additionally, mass outbreaks can occur. In one instance in Houston, TX, these katydids were attracted to lights, flocked to shops, and were a nuisance to the public but did not damage any economically significant crops (Reinhold 1983).
Suggested management techniques, should the situation arise, can be found in the Amblycorypha oblongifolia Featured Creatures article.
Selected References
Beckers OM, Schul J. 2008. Developmental plasticity of mating calls enables acoustic communication in diverse environments. Proceedings of the Royal Society B 275: 1243-1248.
Capinera JL, Scott RD, Walker TJ. 2004. Katydids, pp. 155-160. In Field guide to grasshoppers, katydids, and crickets of the United States. Cornell University Press, Ithaca, USA.
Guagliumi P. 1960. Actual situation of entomology of sugar cane in Venezuela. Proceedings of the International Society of Sugarcane Technologists (10th Congress, Hawaii, 1959): 1000-1010.
Reinhold R. 1983. Houston is at five plagues and counting. The New York Times. (25 September 2015).
SINA. (2014a). Subfamily Copiphorinae: Coneheaded katydids. Singing Insects of North America. University of Florida. (20 September 2015).
SINA. (2014b). Broad-tipped conehead, Neoconocephalus triops (Linnaeus 1758). Singing Insects of North America. University of Florida. (20 September 2015).
SINA. (2014c). Genus Neoconocephalus: Common coneheads. Singing Insects of North America. University of Florida. (24 September 2015).
Walker TJ, Greenfield MD. 1983. Songs and systematics of Caribbean Neoconocephalus (Orthoptera: Tettigoniidae). Transactions of the American Entomological Society 109: 357-389.
Whitesell JJ. 1974. Geographic variation and dimorphisms in song, development, and color in a katydid: Field and laboratory studies (Tettigoniidae, Orthoptera). Ph.D. dissertation. Gainesville, FL: University of Florida. 75 p.
Whitesell JJ, Walker TJ. 1978. Photoperiodically determined dimorphic calling songs in a katydid. Nature 274: 887-888.