The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Three species of mole crickets were inadvertently introduced to the southeastern United States about 1900, and have caused serious plant damage. The introduced species are the shortwinged mole cricket, Neoscapteriscus abbreviatus (Scudder); the southern mole cricket, Neoscapteriscus borellii (Giglio-Tos); and the tawny mole cricket, Neoscapteriscus vicinus (Scudder). These are not the only mole crickets found in North America, but they are the most damaging. For example, a native species, the northern mole cricket, Neocurtilla hexadactyla (Perty), is widely distributed in the eastern states west to about South Dakota and Texas, and including southern Ontario, but is not a pest. The European mole cricket, Gyllotalpa gryllotalpa (Linnaeus), has been introduced from Europe into the northeastern states, but is of minor significance. Changa, Neoscapteriscus didactylus (Latreille), invaded Puerto Rico from South America prior to 1800, and has caused considerable damage to crops on this island, but does not occur elsewhere in the United States.

Credit: Paul M. Choate, University of Florida

Credit: Paul M. Choate, University of Florida

Credit: Washington State Department of Agriculture Archives; www.forestryimages.org
A graphical identification key is available on the Mole Crickets Website. This site also contains detailed information on mole cricket biology, distribution, damage, management (including biological controls) and training tutorials.
Distribution
The shortwinged mole cricket was first observed at Tampa, Florida in 1899 but separate introductions were discovered near Miami in 1902 and Brunswick, Georgia in 1904. The southern mole cricket was similarly introduced to major seaports, beginning with Brunswick in 1904, and followed by Charleston, South Carolina in 1915, then Mobile, Alabama in 1919, and finally Port Arthur, Texas in 1925. The tawny mole cricket, Neoscapteriscus vicinus (Scudder), was first observed at Brunswick, Georgia in 1899. The origin of these crickets is uncertain, but Argentina and Uruguay are likely sources because they occur in these areas of southern South America.
In the years since introduction to the United States, the Neoscapteriscus spp. have expanded their ranges, but they differ considerably in their current distribution. The shortwinged mole cricket, which is flightless, remains fairly confined to the southern Florida and southern Georgia-northeast Florida introduction sites, though it also occurs in Puerto Rico and the Virgin Islands. It has been redistributed in southern Florida, but is largely found in coastal areas. In contrast, the southern mole cricket is now found from North Carolina to Arizona, including the northern regions of Georgia and Alabama and the entire peninsula of Florida, and northern Mexico. Recently it was detected in California (Dillman et al. 2014).The tawny mole cricket is somewhat intermediate in its spread; it occurs from North Carolina to Louisiana, and throughout Florida, but thus far remains restricted to the southern coastal plain.
Life Cycle and Description
The southern and tawny mole cricket are quite similar in appearance and biology. Maturity is attained by the overwintering nymphs in April, and eggs are produced at about this time, usually in April–May. After hatching, nymphs occur through August. Beginning in August or September some adults are found, but overwintering occurs in both the nymphal and adult stages. A single generation per year is normal, though in southern Florida there are two generations in southern mole crickets and an extra peak of adult flight in the summer, resulting in spring, summer, and autumn flights from the two generations (Walker et al. 1983). In both southern and tawny mole crickets, adult emergence occurs earlier in southern Florida than in northern Florida.
The shortwinged mole cricket differs in appearance because of the short wings, but also in behavior because it has no calling song and the short wings render it incapable of flight. As is the case with southern and tawny mole cricket, the eggs may be deposited in April–May, but in southern Florida, the shortwinged mole cricket can produce eggs throughout the year.
Eggs
The eggs are deposited in a chamber in the soil adjacent to one of the tunnels. The chamber is constructed at a depth of 5 to 30 cm below the soil surface. It typically measures 3 to 4 cm in length, width, and height. The eggs are oval to bean-shaped, and initially measure about 3 mm in length and 1.7 mm in width. The eggs increase in size as they absorb water, eventually attaining a length of about 3.9 mm and a width of 2.8 mm. The color varies from grey to brownish. The eggs are deposited in a loose cluster, often numbering 25 to 60 eggs. Duration of the egg stage is 10 to 40 days. Total fecundity is not certain, but more than 100 eggs have been obtained from a single female, and the mean number of egg clutches produced per female is 4.8 (Hayslip 1943).

Credit: Lyle Buss, University of Florida
Nymphs
Hatchlings are whitish initially but turn dark within 24 hours. They may consume the egg shell or cannibalize siblings, but soon dig to the soil surface. The juvenile stages resemble the adults, but have poorly developed wings. The number of instars is variable, probably eight to 10 (Hudson 1987). Nymphs and adults create extensive belowground tunnel systems, usually within the upper 20 to 25 cm of soil. When the soil is moist and warm they tunnel just beneath the surface, but crickets tunnel deeper if the weather becomes cooler or the soil dries. They come to the surface to forage during the evening, usually appearing shortly after dusk if the weather is favorable.
Adults
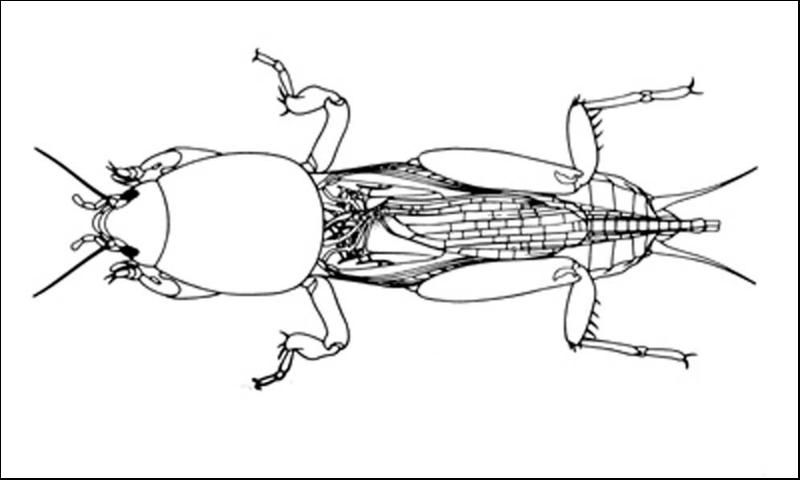
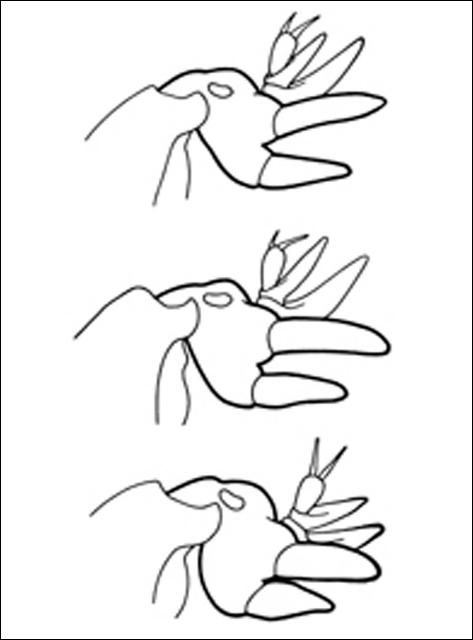
Mole crickets have peculiar enlarged forelegs that are used for digging in the soil. The foretibiae have large blade-like projections, called dactyls, and the number and arrangement of dactyls are used to distinguish among species. These crickets bear antennae that are shorter than their bodies. Females lack a distinct ovipositor. Both sexes have elongate cerci at the tip of the abdomen. The male produces a courtship song that is attractive to females; they normally call during the night. Except for the shortwinged mole cricket, the male enlarges the entrance to his burrow, forming a horn-shaped opening, in preparation for calling. This increases the volume of the call, and allows flying females to locate males. Mating occurs within the male's burrow, after which the burrow may be usurped by the female.
Credit: Susan Wineriter, University of Florida
Credit: Susan Wineriter, University of Florida
The shortwinged mole cricket bears forewings that are shorter than the pronotum. The forewings cover the hind wings, which are very small. The body is mostly whitish or tan in color, although the pronotum is brown, mottled with darker spots. Also, the abdomen is marked with a row of large spots dorsally, and smaller spots dorsolaterally. These crickets measure 22 to 29 mm in length. The two dactyls on the foretibiae are slightly divergent, and separated at the base by a space equal to at least half the basal width of a dactyl. Shortwinged mole cricket makes no calling song, producing only a weak 1 to 5 pulse chirp during courtship.
Credit: Susan Wineriter, University of Florida

Credit: W. C. Adlerz, University of Florida
The southern mole cricket has long hind wings that extend beyond the tip of the abdomen. The forewings are longer than the pronotum, about two-thirds the length of the abdomen. They are broad and rounded at the tips. This cricket is brown in color, with the dorsal surface of the pronotum often quite dark. As with the shortwinged mole cricket, in southern mole cricket the two dactyls on the foretibiae are separated at the base by a space equal to at least half the basal width of a dactyl. Thus, shortwinged and tawny mole crickets are separated by the wing length. The southern mole cricket produces a calling song that consists of a low-pitched ringing trill at about 50 pulses per second. It usually is emitted during the first two hours after sunset.
The tawny mole cricket is quite similar to southern mole cricket in general appearance, with moderately long forewings and long hind wings, a yellowish brown body, and a dark pronotum. It can be distinguished from the southern mole cricket by dactyl form. The tibial dactyls are nearly touching at the base, separated by less than half the basal width of a dactyl. Thus, we distinguish the two long-winged species by the spacing of the dactyls. The tawny mole cricket produces a loud, nasal trill at about 130 pulses per second during the first 90 minutes after sunset.
Summaries of mole cricket life history are given by Worsham and Reed (1912), Thomas (1928), Hayslip (1943), and Walker (1984), though biology of shortwinged mole cricket is poorly documented. Keys to North American and Caribbean-area mole crickets are provided by Nickle and Castner (1984).
Host Plants
Though normally thought of as turf and forage grass pests, mole crickets are omnivorous, feeding on animal as well as plant material. Several studies have indicated that when provided with grass or collected from grass-dominated habitats, the southern mole cricket is less damaging than the tawny mole cricket. The southern mole cricket feeds mostly on other insects, whereas tawny mole cricket is principally herbivorous (Matheny 1981, Matheny et al. 1981, Walker and Ngo 1982). The shortwinged mole cricket also damages grasses but due to its limited range the amount of damage generally is not great. Both the tawny and southern mole crickets are associated with tomato and strawberry fields in Florida (Schuster and Price 1992). Among other vegetable crops reported to be injured are beet, cabbage, cantaloupe, carrot, cauliflower, collard, eggplant, kale, lettuce, onion, pepper, potato, spinach, sweet potato, tomato, and turnip. Other plants injured include chufa, peanut, strawberries, sugar cane, tobacco, and such flowers as coleus, chrysanthemum, and gypsophila. Among the turf grasses, bahiagrass and Bermudagrass are commonly injured by tawny mole cricket, whereas St. Augustinegrass and Bermudagrass are favored by the shortwinged mole cricket. Mole crickets also feed on weeds such as pigweed, Amaranthus spp.
Damage
The crickets usually damage seedlings, feeding aboveground on foliage or stem tissue, and belowground on roots and tubers. Girdling of the stems of seedling plants at the soil surface is a common form of injury, though young plants are sometimes severed and pulled belowground to be consumed. Additional injury to small plants is caused by soil surface tunneling, which may dislodge seedlings or cause them to desiccate. Southern mole cricket does much more tunneling injury than tawny mole cricket.
Grasses differ in susceptibility to injury. Bahiagrass and bermudagrass are especially injured by mole crickets, whether grown as turf grass or as forage, though it is not clear if it is more attractive to crickets or more easily damaged. St. Augustinegrass seem to tolerate injury because of its dense growth habit, but it, too, is injured at times. Centipedegrass and zoysiagrass are infrequently injured.

Credit: W. C. Adlerz, University of Florida
Natural Enemies
Few natural enemies of Neoscapteriscus spp. mole crickets exist naturally in North America. Among the natural enemies are amphibians such as toads, Bufo spp.; birds such as sandhill cranes, Grus canadensis; and mammals such as armadillos, Dasypus novemcinctus. They, and the few predatory insects that attack crickets such as tiger beetles (Coleoptera: Cicindelinae), are not effective. Therefore, several natural enemies have been introduced from South America (Parkman et al. 1996). One introduced parasitoid is Larra bicolor Fabricius (Hymenoptera: Crabronidae), which was imported from Bolivia in 1981 and established in both southern and northern Florida, but seems to be constrained by availability of suitable adult food sources (nectar from flowers) in Florida (Frank et al. 1995). Planting of a favored adult food host, the wildflower Spermacoce spp., helps Larra wasps to survive and attack mole crickets. An entomopathogenic nematode, Steinernema scapterisci Nguyen and Smart, was introduced from Uruguay in 1985 (Nguyen and Smart 1992). It is specific to mole crickets, persists readily under Florida's environmental conditions, and is dispersed by crickets. Field collections consistently show infection levels of 10% or greater (Parkman et al. 1993a, 1993b; Parkman and Smart 1996), and infected crickets die within 12 days. Another parasitoid Ormia depleta (Wiedemann) (Diptera: Tachinidae), which was imported from Brazil in 1988, is attracted to the calls of male mole crickets. Its release has resulted in reduced mole cricket injury in southern Florida (Frank et al. 1996). Marked decline in average abundance of mole crickets in Florida is attributed to the combined effects of these natural enemies, but the crickets remain capable of causing damage under certain conditions. Other nematode species as well as fungi are sometimes marketed for mole cricket suppression and can provide temporary relief from mole cricket injury, but they are a temporary solution. Unlike the other natural enemies mentioned here, they do not persist once applied.
Management
Sampling
Various methods have been developed to estimate mole cricket populations. A commonly used, but not particularly reliable technique, is the assessment of population density by the frequency of soil surface tunneling. Tunneling is affected by soil moisture levels, and is most appropriate for nymphs. The ability to detect tunneling is seriously affected by the amount of vegetation, so though tunneling can be detected easily in crops and bahia lawns and pastures, tunneling is not really discernable in St. Augustinegrass. A more consistent but labor intensive approach for estimation of nymph and adult abundance is flushing with about a 0.5% aqueous solution of dishwashing soap. Soil flushing is affected by soil moisture conditions, with greater extraction efficiency as the soil approaches field capacity (Hudson 1989).
On turfgrass the usual recommendation for soap flushing is to apply 1.5 oz of liquid dishwashing soap in 2 gal of water with a sprinkling can to 4 sq ft of turf (60 ml of soap in 7.6 liters of water to 0.4 m sq). If two to four mole crickets come to the soil surface within three minutes of application of the soap solution, corrective action is justified to reduce mole cricket numbers. Flushing with synergized pyrethrin insecticide solution is equally effective (Hudson 1988). A soil washing apparatus also has been developed to separate crickets from soil (Fritz 1983). Adult females can be captured with sound traps that use electronic sound synthesizers to lure crickets to a catching device, usually a large funnel.
Insecticides
Liquid and granular formulations of insecticides are commonly applied to the soil to suppress mole crickets. In some cases, insecticide application should be followed by irrigation because the insecticide must enter the root zone of the plants to be most effective, but this is an insecticide-specific requirement so the insecticide label should be read carefully for application directions. Bait formulations are also useful. Various baits have proven effective, but most contain wheat bran, cottonseed meal, or some other grain product plus 2-5% toxicant. Addition of 5 to 15% water and 2 to 5% molasses to the grain-toxicant mixture are sometimes recommended (Thomas 1928, Walker 1984). Mole crickets feed at night so baits should be applied in the early evening. Baits are incompatible with irrigation and rainfall.
Insect Management Guide for Turf Insects
Host Plant Resistance
Efforts have been made to find turf and pasture grass varieties that are resistant to attack by mole crickets. If grass varieties contain antibiotic properties, or otherwise limit the reproductive abilities of mole crickets, this can translate into fewer crickets. Thus far, strains have been identified which are fairly tolerant of feeding or which are not preferred by mole crickets, primarily the finer textured grass selections, but considerable improvement in these grasses is needed before they can affect cricket population biology.
Biological Control
Biological control of mole crickets can be enhanced by the application of the entomopathogenic nematode Steinernema scapterisci and possibly to a lesser degree by other entomopathogenic nematodes. This nematode can be purchased from commercial suppliers, sprayed as a suspension in water to soil, and is fairly persistent in the soil. It is more effective when applied to adults than when applied to nymphs. In areas where parasitism of crickets by insects is low, the South American parasitoids mentioned previously under "natural enemies" can be introduced.
The long-term benefits of mole cricket biological in pastures are quite impressive. Economic analysis of the benefits to ranchers in Florida accruing from the introduction of biological control organisms suggests a savings of $136 million over a 10-year period (Mhina et al. 2016).
Selected References
Braman SK, Pendley AF, Carrow RN, Engelke MC. 1994. Potential resistance in zoysiagrasses to tawny mole crickets (Orthoptera: Gryllotalpidae). Florida Entomologist 77: 301–305.
Capinera, J.L. 2001. Handbook of Vegetable Pests. Academic Press, San Diego. 729 pp.
Dillman AR, Conin CJ, Tang J, Gray DA, Sternberg PW. 2014 A modified cricket lure and description of Scapteriscus borelli (Orthoptera: Gryllotalpidae) range expansion and calling song in California. Environmental Entomology 43: 146–156.
Fasulo T.R. (2002). Mole Crickets and Enemies of Mole Crickets. Bug Tutorials. UF/IFAS. CD-ROM. SW170.
Fowler, H.G. 1987. Field behavior of Euphasiopteryx depleta (Diptera: Tachinidae): phonotactically orienting parasitoids of mole crickets (Orthoptera: Gryllotalpidae: Scapteriscus). J New York Entomol Soc 95: 474–480.
Frank, J.H. 1994. Biological control of pest mole crickets. Pages 343–352 in D. Rosen, F.D. Bennet, and J.L. Capinera (eds.) Pest Management in the Subtropics. Biological Control - A Florida Perspective. Intercept, Andover, England.
Frank, J.H., T.R. Fasulo, D.E. Short and A.S. Weed (2002). MCricket: Alternative Methods of Mole Cricket Control: A knowledgebase and tutorial on mole crickets and their control. (Version 2.0). UF/IFAS. SW-89.
Frank, J.H. and J.P. Parkman. 1999. Integrated pest management of pest mole crickets with emphasis on the southeastern USA. Integr Pest Manage Rev 4: 39–52.
Frank, J.H., T.J. Walker, and J.P. Parkman. 1996. The introduction, establishment, and spread of Ormia depleta in Florida. Biol Control 6: 368–377.
Frank, J.H., J.P. Parkman, and F.D. Bennett. 1995. Larra bicolor (Hymenoptera: Sphecidae), a biological control agent of Scapteriscus mole crickets (Orthoptera: Gryllotalpidae), established in northern Florida. Florida Entomologist 78: 619–623.
Fritz, G.N. 1983. A technique for separating mole crickets from soil. Florida Entomologist 66: 360–362.
Hayslip, N.C. 1943. Notes on biological studies of mole crickets at Plant City, Florida. Florida Entomologist 26: 33–46.
Hudson, W.G. 1987. Variability in development of Scapteriscus acletus (Orthoptera: Gryllotalpidae). Florida Entomologist 70: 403–404.
Hudson, W.G. 1988. Field sampling of mole crickets (Orthoptera: Gryllotalpidae: Scapteriscus): a comparison of techniques. Florida Entomologist 71: 214–216.
Hudson, W.G. 1989. Field sampling and population estimation of the tawny mole cricket (Orthoptera: Gryllotalpidae). Florida Entomologist 72: 337–343.
Matheny, Jr., E.L. 1981. Contrasting feeding habits of pest mole cricket species. J Econ Entomol 74: 444–445.
Matheny, Jr., E.L., A. Tsedeke, and B.J. Smittle. 1981. Feeding response of mole cricket nymphs (Orthoptera: Gryllotalpidae: Scapteriscus) to radiolabeled grasses with, and without, alternative foods available. J Georgia Entomol Soc 16: 492–495.
Mhina G.J., Leppla N.C., Thomas M.H., Solís D. 2016. Cost effectiveness of biological control of invasive mole crickets in Florida pastures. Biological Control 100: 108–115.
Nickle, D.A. and J.L. Castner. 1984. Introduced species of mole crickets in the United States, Puerto Rico, and the Virgin Islands (Orthoptera: Gryllotalpidae). Ann Entomol Soc Am 77: 450–465.
Nguyen, K.B. and G.C. Smart, Jr. 1992. Life cycle of Steinernema scapterisci Nguyen & Smart, 1990. J Nematol 24:160–169.
Parkman, J.P. and G.C. Smart, Jr. 1996. Entomopathogenic nematodes, a case study: introduction of Steinernema scapterisci in Florida. Biocontrol Sci Tech 6: 413–419.
Parkman, J.P., J.H. Frank. K.B. Nguyen, and G.C. Smart, Jr. 1993b. Dispersal of Steinernema scapterisci (Rhabditida: Steinernematidae) after inoculative applications for mole cricket (Orthoptera: Gryllotalpidae) control in pastures. Biol Control 3: 226–232.
Parkman, J.P., J.H. Frank, T.J. Walker, and D.J. Schuster. 1996. Classical biological control of Scapteriscus spp. (Orthoptera: Gryllotalpidae) in Florida. Environ Entomol 25: 1415–1420.
Parkman, J.P., W.G. Hudson, J.H. Frank, K.B. Nguyen, and G.C. Smart, Jr. 1993a. Establishment and persistence of Steinernema scapterisci (Rhabditida: Steinernematidae) in field populations of Scapteriscus spp. mole crickets (Orthoptera: Gryllotalpidae). J Entomol Sci 28: 182–190.
Poe, S.L. 1976. Reinfestation of treated tomato fields by mole crickets. Florida Entomologist 59: 88.
Schuster, D.J. and J.F. Price. 1992. Seedling feeding damage and preference of Scapteriscus spp. mole crickets (Orthoptera: Gryllotalpidae) associated with horticultural crops in west-central Florida. Florida Entomologist 75: 115–119.
Thomas, W.A. 1928. The Porto Rican mole cricket. USDA Farmers' Bull 1561. 8 pp.
Walker, T.J. 1982. Sound traps for sampling mole cricket flights (Orthoptera: Gryllotalpidae: Scapteriscus). Florida Entomologist 65: 105–110.
Walker, T.J. (ed.). 1985. Mole crickets in Florida. Florida Agric Exp Stn Bull 846. 54 pp.
Walker, T.J. and D. Ngo. 1982. Mole crickets and pasture grasses: damage by Scapteriscus vicinus, but not by S. acletus (Orthoptera: Gryllotalpidae). Florida Entomologist 65: 300-306.
Walker, T.J., J.A. Reinert, and D.J. Schuster. 1983. Geographical variation in flights of mole crickets, Scapteriscus spp. (Orthoptera: Gryllotalpidae). Ann Entomol Soc Am 76: 507–517.
Worsham, E.L. and W.V. Reed. 1912. The mole cricket (Scapteriscus didactylus Latr.). Georgia Agric Exp Stn Bull 101. 263 pp.