Pests, primarily insects and diseases, are common and important problems for pepper plants regardless of variety. To effectively manage the pests, Integrated Pest Management (IPM), including host resistance, cultivation, sanitation, and physical, mechanical, biological, and chemical approaches, should be implemented. Pest management of peppers in Miami-Dade County is challenging because of a climate favorable to pests. To assist local pepper growers in maintaining crop productivity and improving the quality of produce, this publication illustrates common pests including major diseases and insects and their effective management. The targeted audiences are pepper growers, pest control operators, extension agents and specialists, crop advisors, industrial personnel, and graduate students.
Major Diseases and Control Measures
The major diseases of pepper plants grown in Miami-Dade County are damping off, bacterial spot, Phytophthora blight, anthracnose, and some viruses.
Damping off: Damping off is caused by any of several pathogens during the seedling stage, especially soil-borne fungi such as Phytophthora capsici, Rhizoctonia solani, Pythium spp., and Fusarium spp. After planting, pepper seeds can be attacked by these pathogens, resulting in poor germination. Excessive irrigation before emergence may exacerbate this problem, resulting in a high incidence of infected plants. Raising transplants in sterile soils or potting mixes instead of direct seeding is recommended. During germination and the young seedling stage, avoidance of overwatering and providing good drainage can help seed germination and healthy seedling development. In addition, treating seeds with fungicides such as thiram can help protect pre-emergent seedlings from damping off.
Bacterial leaf spot (Figure 1) appears as circular to irregular water-soaked lesions on leaves and stems of pepper plants. As the lesion develops it becomes purplish-gray with a black center and is surrounded by a narrow yellow halo. Symptoms tend to be more severe in the lower canopy, where infected leaves become ragged and eventually turn brown and fall off, resulting in severe defoliation of the plants (Figure 2). Pepper flowers and fruits also can be infected by the bacteria, which usually causes blossom drop and small, dark, scabby lesions on the fruit.

Credit: Shouan Zhang

Credit: Shouan Zhang
To control bacterial spot, use plant varieties that are resistant to the disease, and use disease-free seed for transplants. Rotate crop species in the fields to avoid infection of future pepper fields by carryover bacteria on crop residues. Avoid planting peppers after tomatoes, and tomatoes should never follow peppers. Remove plant debris, weeds in the Solanaceae family such as black nightshade, and volunteer pepper plants. Treating seeds in a 20 percent bleach solution for 40 minutes followed by rinsing with water has proven to be successful in areas with a history of bacterial spot. Actigard (acibenzolar-S-methyl), a plant defense activator, and copper-based chemicals such as copper hydroxide and copper sulfate mixed with mancozeb/maneb can be applied to control the disease.
Phytophthora blight: Phytophthora blight, caused by a soil-borne, fungus-like oomycete, Phytophthora capsici, can severely affect the growth of pepper plants by causing them to wilt and eventually die (Figure 3). Similar to fungal pathogens, Phytophthora causes disease when the soil becomes excessively wet, either from over-irrigation or from heavy rain. Therefore Phytophthora disease outbreaks usually occur in heavy soil or in low spots where water tends to accumulate for long periods. Diseased plants are commonly clustered within fields, such as in specific rows or at the end of the field, while the remainder of the planting often appears healthy. When the disease occurs in certain rows, it often indicates excessive irrigation and the spread of infective spores by water.

Credit: Qingren Wang, UF/IFAS
Phytophthora capsici also causes other symptoms, such as spots, blight, and fruit rot (Figure 4). The disease often spreads from rain splashing infested soil onto plants. Hence the stems, leaves, and fruit often become infected when wet conditions persist for several days.

Credit: Shouan Zhang
Phytophthora capsici can often survive in crop debris and soil as oospores, which may persist for a few years even in the absence of the host. Therefore crops should be rotated away from peppers or other susceptible hosts, such as tomato and eggplants. Suggested rotational crops include cabbage and onion.
Several chemicals are registered to control Phytophthora blight. Metalaxly is registered for use on peppers to control root and crown rots; however, once the plant is infested, metalaxyl is not very effective in helping the plants recover (Goldberg 2001). Copper applied alone or in combination with other fungicides may provide protection against the foliar blight associated with the disease. Recently, fluopicolide has proven to be effective in Phytophthora blight control. However, no fungicides are highly effective in controlling this disease under favorable climatic conditions. Furthermore, resistance to fungicides including metalaxyl or mefenoxam has been reported in some P. capsici strains. Therefore resistance-management strategies could be chosen carefully to avoid developing resistance to P. capsici.
Rhizoctonia root rot: Rhizoctonia solani is a common soil fungus that infects many kinds of vegetables including peppers. The pathogen may cause root rot of both seedlings and mature plants (Figure 5). Symptoms of the disease usually appear on the lowest part of the main stem near soil level. As the fungus moves up and down the stem and the tap root decays, the developing lesion turns reddish-brown, which is a key diagnostic feature of the disease. Once the plant is infected, the vigor is greatly reduced, the yield is poor, and the plant wilts and eventually dies.

Credit: Qingren Wang, UF/IFAS
There currently is no fungicide that can effectively control this disease once the plant becomes infected. Prevention may be the only practical strategy. To reduce the risk of root rot in pepper plants, young plants should be watered shallowly and frequently until they reach 8 to 12 inches tall. Drip irrigation keeps the leaves dry, which helps prevent the spread of the disease. If using overhead irrigation, water the pepper plants early in the morning so that leaves dry off quickly, and avoid working in the field when it is wet. Promptly remove infected plants before the disease has a chance to spread. Rotation of pepper plants with non-host crops is always advised.
There currently is no fungicide that can effectively control this disease once the plant becomes infected. Prevention may be the only practical strategy. To reduce the risk of root rot in pepper plants, young plants should be watered shallowly and frequently until they reach 8 to 12 inches tall. Drip irrigation keeps the leaves dry, which helps prevent the spread of the disease. If using overhead irrigation, water the pepper plants early in the morning so that leaves dry off quickly, and avoid working in the field when it is wet. Promptly remove infected plants before the disease has a chance to spread. Rotation of pepper plants with non-host crops is always advised.
Anthracnose: Anthracnose (also called ripe rot) is caused by Colletotrichum spp. fungi, and it is a common disease, especially with overhead irrigation. The fungus persists in infected seed, crop debris, and alternate hosts. The infection often occurs during periods of excessive irrigation or rainfall on immature fruit; however, symptoms normally are not expressed until the fruits become mature and change to their final color. Symptoms of anthracnose first appear as small, water-soaked lesions, which expand rapidly. Fully expanded lesions are sunken and range in color from dark red to tan to black. As the infection progresses, small buff or salmon-colored spots appear either scattered or in concentric rings within the lesions. Because Colletotrichum spp. attacks immature fruit, infection occurs in the field, but the symptoms often do not develop until after harvest.
To control anthracnose, it is important to use clean seed and to rotate crops. Fungicide sprays may be helpful during periods of favorable environmental conditions. Several registered fungicides are available to control the disease, such as Mankocide (copper hydroxide and manozeb), fungicides containing chlorothalonil, Fontelis (penthiopyrad), Cabrio 2.08 F (pyraclostrobin), and Quadris Top (azoxystrobin plus difenoconazole) (Ozores-Hampton et al. 2014).
Cucumber mosaic virus: The symptoms of Cucumber mosaic virus (CMV) vary considerably depending on the viral strain, but most plants develop a narrowing of leaves in addition to stunting, yellowing, and whitish spots on the leaves (Figure 6). More than 80 species of aphids are capable of transmitting the virus. Insecticides registered for pepper plants, such as Admire Pro (imidacloprid), Beleaf 50 SG (flonicamid), and Movento (spirotetramat), are used to control aphids, which vector CMV. Rotation with crops other than susceptible hosts (such as tomatoes, lettuce, and cucurbits) also can help control CMV.

Credit: Qingren Wang
Tobacco mosaic virus: Tobacco mosaic virus (TMV), one of the most common and widespread plant viruses, is seed borne. The most common symptoms on pepper plants are raised bumps and mottled light and dark green areas on the foliage. TMV is very stable and can survive in plant debris for many years. The virus is readily transmitted mechanically by dirty hands, cutting tools, and other equipment, but not by any known insect, nematode, or fungal vector.
Selection of disease-resistant varieties or disease-free seeds is important for controlling TMV. In the 1960s much of the cultivated tabasco peppers were destroyed by TMV. The first resistant variety, 'Greenleaf Tabasco', was not grown commercially until about 1970 (Andrews 1998). Peppers should not be planted in fields with a history of TMV. Sanitation is important for control, such as minimizing the handling of plants and washing hands and equipment with disinfectants before touching healthy plants.
Major Insects and their Control
Pepper weevil: The pepper weevil (PW), Anthonomus eugenii Cano, is the worst biotic constraint on the production of peppers (Capsicum spp.) in Florida, California, Texas, Mexico, Central America, and the Caribbean. Feeding by larvae and adults can cause severe losses in both yield and fruit quality.
To attack the plant, a female adult weevil (Figure 7) punctures the flower buds or young fruit of peppers with her mouthparts, lays eggs, and then seals the puncture containing the eggs with a light brown fluid, which hardens and darkens. The larval stage of PW, commonly known as a grub (Figure 8), is white or gray with a yellowish brown head. PW larvae feed on pepper and other plants in the Solanaceae family, such as the common black nightshade weed, Solanum americanum, and the tomatillo, Physalis philadelphica. All varieties of pepper are susceptible to PW attack, whereas tomatillos are only moderately susceptible.

Credit: Dakshina Seal
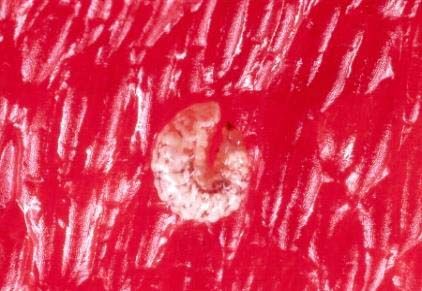
Credit: Dakshina Seal
Oviposition damage from pepper weevils causes the fruits, flowers, and buds to fall off prematurely, which is very common and the most obvious sign of infestation. Larval feeding within the mature fruit results in their cores becoming brown or moldy, which seriously reduces pepper quality. Occasionally, infestation by the less common Cuban pepper weevil (Faustinus cubae Boheman) also causes large yield losses in pepper crops (Capinera 2011).
Cultural practices are effective in suppressing PW populations. Physical or mechanical measures, such as sanitation and the removal and destruction of infested fruits and alternate hosts can help disrupt the PW life cycle and considerably reduce infestation levels. The use of yellow-sticky and pheromone traps and attract-and-kill methods for pepper weevils can not only reduce the adult populations but also help in monitoring for PWs in the field. Insecticides are an important tool in controlling PW (Seal and Schuster 1995). However, insecticidal applications should begin one week before flowering. Registered insecticides for pepper weevil control include Actara®(thiamethoxam), Pounce® 25 W (Permethrin), Prokil Cryolite® 96 (Cryolite), and Vydate® L (Oxamyl). For more information on the selection, use, rotation, and application of these insecticides, consult the Vegetable Production Handbook for Florida 2014–2015, Chapter 8 (Ozores-Hampton et al. 2014).
Silverleaf whitefly: The silverleaf whitefly (SLW), Bemisia argentifolii (Bellows and Perring) (Figure 9), is a common pest of all vegetable crops in southern Florida, including Miami-Dade County, nearly all year long. SLW typically is a minor pest of pepper, but occasionally it can be serious late in the growing season. The adult SLW is about 1/16 inch long with white, powdery wings held flat over the yellow body when at rest. Whiteflies often are found in pairs on the undersides of leaves, and adult females lay most of their eggs on young leaves. The life cycle from egg to adult can be as short as two weeks under favorable weather conditions in the subtropical climate of Miami-Dade County.

Credit: Dakshina Seal
Whiteflies feed on sap from the plant vascular system (phloem) through a needle-like mouthpart known as a stylet, and they simultaneously excrete a liquid substance known as honeydew, which can serve as a food for sooty mold. Heavy growth of sooty mold forms a dark layer that reduces penetration of sunlight, thus decreasing photosynthesis and plant vigor. Adult whiteflies often transmit plant viruses from infected to healthy plants, including cucumber mosaic virus (CMV) on pepper plants, which usually causes an outbreak of pepper virus. Once a whitefly acquires the virus, it may be transmitted for the rest of the fly's life.
To control whiteflies, cultural practices, such as disrupting its life cycle by removal of infested host plants and plant residues, may help control the pest. Various synthetic biorational and chemical insecticides are effective in controlling whiteflies (Seal 1993; Seal et al. 1994). Insecticides registered and available for whitefly control include neonicotinoids, diamides, Neemix® (azadirachtin), Requiem® (chenopodium ambrosioides), and Movento®(spirotetramat). Further information on chemical insecticides is available in Chapter 8, "Pepper Production," in the Vegetable and Small Fruit Production Handbook of Florida (Ozores-Hampton et al. 2014).
Broad mite: The broad mite, Polyphagotarsonemus latus (Banks), has eight legs and is almost microscopic (less than 0.2 millimeter long) and difficult to see with the naked eyes. It usually occurs on the underside of young, emergent leaves. In peppers, broad mites feed on all above-ground plant tissues and cause leaves to become thick and narrow, which give them a "strappy" appearance (Figure 10). Heavy feeding may lead to flower abortion and stunted plants. High temperatures and humidity often increase population growth rates, though in Miami-Dade County broad mite damage to pepper plants commonly occurs during winter and spring.

Credit: Qingren Wang
Broad mites spread through movement of plant materials, farm equipment, birds, insects, and mammals. Limiting the movement of these materials and organisms can reduce mite populations. Destruction of infested plant parts can help reduce the spread of mites to nearby plants. In addition to cultural practices, insecticides, such as Agrimek® (abamectin), Portal® (fenpyroximate), Neemix®, and Requiem® are effective in controlling broad mite populations on pepper. Organic alternatives, such as insecticidal soap and mineral oil, also can effectively control broad mites.
Green Peach Aphids (GPA): The green peach aphid (GPA), Myzus persicae (Sulzer), is an important pest of peppers. Green peach aphid populations can increase rapidly on pepper plants because of the short generation time (10–13 days). In south Florida, wingless and winged forms are all female and give birth to live nymphs. Aphids damage pepper plants by sucking the sap with their stylets. Heavy aphid infestations may cause distorted leaves and stunted plants. Aphids feeding on flowers can reduce the fruit set and may transmit viruses from infected to healthy plants.
Registered insecticides, such as Admire Pro® (imidacloprid), Assail 70 WP® (acetamiprid), Vydate® (oxamyl), Lannate® (methomyl), and Requiem®, are available for control of GPA on pepper crops (Ozores-Hampton 2014). Aphids commonly occur in pepper and other crops, but purposely controlling the pests is uncommon because most control programs for other pests also control aphids.
Armyworms: The beet armyworm, Spodoptera exigua (Hübner), is a common pest of peppers in south Florida including Miami-Dade County, where Southern Armyworm (Spodoptera eridania) also can occasionally infest pepper plants. Armyworm adults have wingspan of about 1.5 inches, with the front wing streaked with creamy, gray, light brown, and black and the hind wing white with some dark color on the margins. Larvae are dark caterpillars with two yellowish lateral lines interrupted by a large dark spot on the first abdominal segment. Armyworm larvae can severely damage pepper leaves and fruits by their voracious feeding habits (Figures 11, 12).

Credit: Qingren Wang

Credit: Dakshina Seal
The beet armyworm has a wide host range, including bean, cabbage, corn, cowpea, eggplant, lettuce, pepper, potato, sweet potato, tomato, and turnip. Field crops damaged by beet armyworms may include alfalfa, corn, cotton, peanut, safflower, sorghum, soybean, sugar beet, and tobacco. Various weeds growing in and around pepper fields serve as hosts for beet armyworms, including lambsquarters, Chenopodium album; mullein, Verbascum sp.; pigweed, Amaranthus spp.; purslane, Portulaca oleracea; Russian thistle, Salsola kali; parthenium, Parthenium hysterophorus; and tidestromia, Tidestromia sp. Therefore removal of host crop debris and weeds is very important in effectively controlling beet armyworms on peppers.
In addition, cultural and physical practices are important for reducing armyworm populations, including hand capture (on a small scale), rotation of peppers with non-host crops, using attract-and-kill techniques, and keeping the field free from weed hosts.
For chemical control, Bacillus thuringiensis-based insecticides are highly effective in reducing first and second instar armyworm larvae. The use of Neemix® and insecticidal soap as an organic alternative to conventional pesticides also can suppress the early stages of armyworms. Once mature larvae appear in the field, registered insecticides available for armyworm control include Radiant® (spinetoram), Avaunt® (indoxacarb), Confirm® (tebufenozide), Rimon® (novaluron), Coragen® (chlorantraniliprole), and Belt® (flubendiamide).
Thrips: In south Florida Thrips palmi Karny has been reported as a devastating pest of bean, squash, cucumber, eggplant, pepper, potato, and okra (Seal and Baranowski 1992). Thrips palmi is a polyphagous and economically very important pest (Kirk 1997). The wide host range of T. palmi includes at least 50 plant species (Wang and Chu 1986) and provides abundant food sources allowing it to survive throughout the year. Regardless of host plant in south Florida, T. palmi populations are abundant from January to March and decrease after May, possibly because of the rainy season lasting from mid-May to mid-September (Seal 1997). Thrips palmi attacks all above-ground parts of pepper plants and causes serious economic damage even at low population levels. A single larva feeding under the calyx of a pepper fruit can render it unmarketable.
Thrips palmi is a small insect, only about 1.0 mm long (Figure 13), with four developmental stages: egg, larva, pupa, and adult. Eggs are laid within plant tissues and hatch in 3–6 days to become larvae, which feed on the plants until pupation. The larval period lasts 5–15 days, depending on temperature, followed by the pupal stage, which occurs within the soil and lasts 3–14 days. The entire developmental period from egg to adult requires 2–3 weeks, and adult longevity is about 2–4 weeks (Seal 1997).

Credit: Dakshina Seal
Cultural practices are important in managing thrips, such as repeatedly disking soil to kill pupae of T. Palmi by exposure to sunlight, destroying weed hosts in and near the crop, avoidance of planting peppers in or near an infested field, and maintaining a host-free period to disrupt the thrips life cycle.
Biological control can be effective under specific circumstances. For example, the minute pirate bug, Orius insidiosus Say, is a native, very active thrips predator. Throughout the life cycle of a minute pirate bug one nymph or adult can feed on 10–25 thrips larvae per day (Seal 1997).
Chemical insecticides of different classes and modes of action commonly are used by commercial growers to control T. palmi, but only with limited success. Most of the insecticides, such as Lannate®, Vydate®, and Agri-Mek®, can provide 45–60 percent reduction of T. palmi populations (Seal et al. 2013). However, fermentation products such as Spintor® and Radiant® have been very effective in managing T. palmi compared to other kinds of insecticides. The botanical insecticides, Neemix® and Requiem®, are benign to the natural enemies yet can suppress thrips populations. Nonetheless, the use of insecticides of various modes of action should be rotated to avoid the development of T. palmi resistance for any specific insecticide.
Two other thrips species, Frankliniella occidentalis (Pergande) and F. schultzei Trybom, or flower thrips, also are pests of peppers in southern Florida. They often cause serious economic damage by transmitting viral diseases late in the growing season. Even at low population levels, such as one adult per 10 plants, they can transmit viruses to 10–15 plants (Seal et al. 2014). The developmental biology and the recommended methods for cultural, biological, and chemical control of flower thrips are similar to those for melon thrips, T. palmi.
American serpentine leafminer: The American serpentine leafminer (Liriomyza trifolii) is considered a key pest of pepper and other vegetable crops. It causes damage by mining and feeding punctures in host leaves. Stippling and mining considerably reduces photosynthetic rates and mesophyll and stomatal conductance, which seriously affects plant growth and may kill the plant (Parrella et al. 1985). Severe mining kills seedlings and causes premature leaf drop, which leads to sunburn in tomatoes. Punctures caused by the stippling and mining of leafminers often provide entry points for fungal and bacterial diseases.
Naturally occurring biological control agents, such as parasitic wasps, are very effective in suppressing leaf miners. To increase natural enemy populations, avoid broad spectrum insecticides, which often kill predators along with the target pests. Chemical insecticides that are effective in controlling leafminers include Agri-Mek® (abamectin), Radiant®, Spintor®, Coragen®, and some neonicotinoids.
Conclusion
Management of pepper pests by implementing Integrated Pest Management (IPM) techniques including physical, mechanical, biological, sanitation, cultural, and chemical measures are recommended by the use of scouting and then comparing the results to economically based thresholds of potential pest damage. However, prevention is always preferable to suppression of pests because prevention typically saves money and time. Prevention involves using cultural, physical, mechanical, sanitary, and biological approaches. For example, selecting pest-free seeds or transplants is essential. Preparing land with laser leveling is important because too much water stuck in low areas often causes plant pathogens to thrive, especially fungi and bacteria. Recommended practices include promptly removing weeds and crop debris to destroy hosts of pests, raising beds and fumigating the soil before planting, and choosing pest-resistant varieties or cultivars for prevention of pest outbreaks. If a chemical procedure appears to be the best choice, it is very important to identify the pests correctly by scouting, then select and apply the pesticide(s) recommended to control the specific pest and certified for peppers by following the label. To avoid the development of resistance to pesticides (Andrews 1998), it is critical to rotate pesticides with different modes of action.
Reference
Andrews, J. 1998. The Pepper Lady's Pocket Pepper Primer. University of Texas Press.
Berdegue, M., M. K. Harris, D. W. Riley, and B. Villalon. 1994. "Host plant resistance on pepper to the pepper weevil, Anthonomus eugenii Cano." Southwestern Entomologist 19: 265–271.
Boyd, E. J. McAvoy, H. H. Smith, and G. E. Vallad. 2014. "Pepper Production." In Vegetable and Small Fruit Production Handbook of Florida (2014–2015), 137–150.
Capinera, J. L. 2011. "Pepper weevil, Anthonomus eugenii Cano (insecta: Coleoptera: Curculionidae)." EENY-278. Gainesville, FL: University of Florida Institute of Food and Agricultural Sciences, http://entomology.ifas.ufl.edu/creatures.
Goldberg, N. P. 2001. "Chile pepper diseases." Circular 549. New Mexico State University. https://aces.nmsu.edu/pubs/_circulars/CR549/welcome.html.
Kirk, W. D. J. 1997. "Feeding." In Thrips as crop pests, edited by T. Lewis, 119–174. Wallingford, UK: CAB International.
Parrella, M. P. and V. P. Jones. 1985. "Yellow traps as monitoring tools for Liriomyza trifolii (Diptera: Agromyzidae) in chrysanthemum greenhouses." J. Econ. Entomol. 78: 53–56.
Seal, D. R. 1993. "Effectiveness of Different Insecticides for the Control of Sweetpotato Whitefly, Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) on Vegetable Crops in Southern Florida." Proc. Fla. State Hort. Soc. 106: 224–228.
———. 1997. "Management and Biology of Thrips palmi Karny (Thysanoptera: Thripidae)." In New Development in Entomology, edited by K. Bondarfi, 161–181.Trivandrum, India: Research Signpost.
Seal, D. R. and R. M. Baranowsky. 1992. "Effectiveness of Different Insecticides for the Control of Melon Thrips, Thrips palmi Karny (Thysanoptera: Thripidae) Affecting Vegetables in South Florida." Proc. Fla. State Hort. Soc. 105: 315–319.
Seal, D. R., J. H. Bryan, and H. D. Bryan. 1994. "Abundance and Management of Silverleaf Whitefly, Bemisia argentifolii (Homoptera: Aleyrodidae), on Ornamentals." Proc. Fla. State Hort. Soc. 107: 222–226.
Seal, D. R., V. Kumar, G. Kakkar, and S. C. Mello. 2014. "Common Blossom Thrips, Frankliniella schultzei (Thysanoptera: Thripidae) Management and Groundnut Ring Spot Virus Prevention on Tomato and Pepper in Southern Florida." Florida Entomol. 97: 374–383.
Seal, D. R. and D. J. Schuster. 1995. "Control of Pepper Weevil, Anthonomus eugenii, in West-Central and South Florida." Proc. Fla. Hort. Soc. 108: 220–225.
Wang, C. L., and Y. I. Chu. 1986. "Rearing Method of Southern Yellow Thrips, Thrips palmi Karny in Laboratory." Plant Protection Bulletin 28: 411.