Prescribed Fire Size, Patchiness, and Pyrodiversity in the Southeastern United States
Introduction
Variation in prescribed fire characteristics over space and time can create a fire regime that is beneficial for many different plants and animals. Prescribed fire regimes are described by frequency, seasonality, intensity, size, and heterogeneity. When prescribed fire characteristics vary over time, the variability in the fire regime causes variation across the landscape (Table 1). Consider fire size: suppose fires often burn only small patches of vegetation in an area, but in some years, they completely burn all the vegetation in an area. In that case, there will be areas of vegetation on the landscape that vary in structure over time. This variation in vegetation structure affects biodiversity because plants and animals use resources (e.g., food, pollinators, mates, nest materials, soil microbes, etc.) at different times of the year in different types of vegetation patches. For example, deer and turkeys might use recently burned areas to search for food, while birds might find good nesting habitats in unburned areas (Fill and Crandall 2018).
Pyrodiversity refers to variation in fire regimes and their ecological effects over time and space. Highly variable fire regimes are said to have high pyrodiversity, and more consistent fire regimes have lower pyrodiversity (Jones and Tingley 2021). The original definition of pyrodiversity proposed that more variable fire regimes can meet more habitat needs, thereby increasing biodiversity (Martin and Sapsis 1992). The beneficial aspects of fire regime variability are used in other definitions for pyrodiversity and several related concepts (see the list of definitions below). The purpose of this publication is to provide information on how variation in prescribed fire size and heterogeneity and their impact over time relate to the habitat needs of animals and plants that live in frequently burned savannas. This document’s target audience consists of southeastern United States fire managers, land managers, and those interested in the ecological effects of prescribed fire.
Prescribed Fire Size
The size and arrangement of burned areas influence pyrodiversity by affecting resources (Figure 1). The amount of land an animal or plant uses to meet its needs is called the organism’s home range (Powell and Mitchell 2012). When the size of a prescribed fire is much larger than a home range, an organism’s habitat needs must be met within the burned area. When the size of a fire is smaller than a home range, an organism could ideally move between burned and unburned areas to find resources. This concept applies to both horizontal and vertical space (Wiens 1976). For example, scorched trees provide a different habitat than trees with green leaves. This fact sheet focuses on horizontal spatial scale because most fires in southeastern landscapes are unlikely to burn into the treetops.
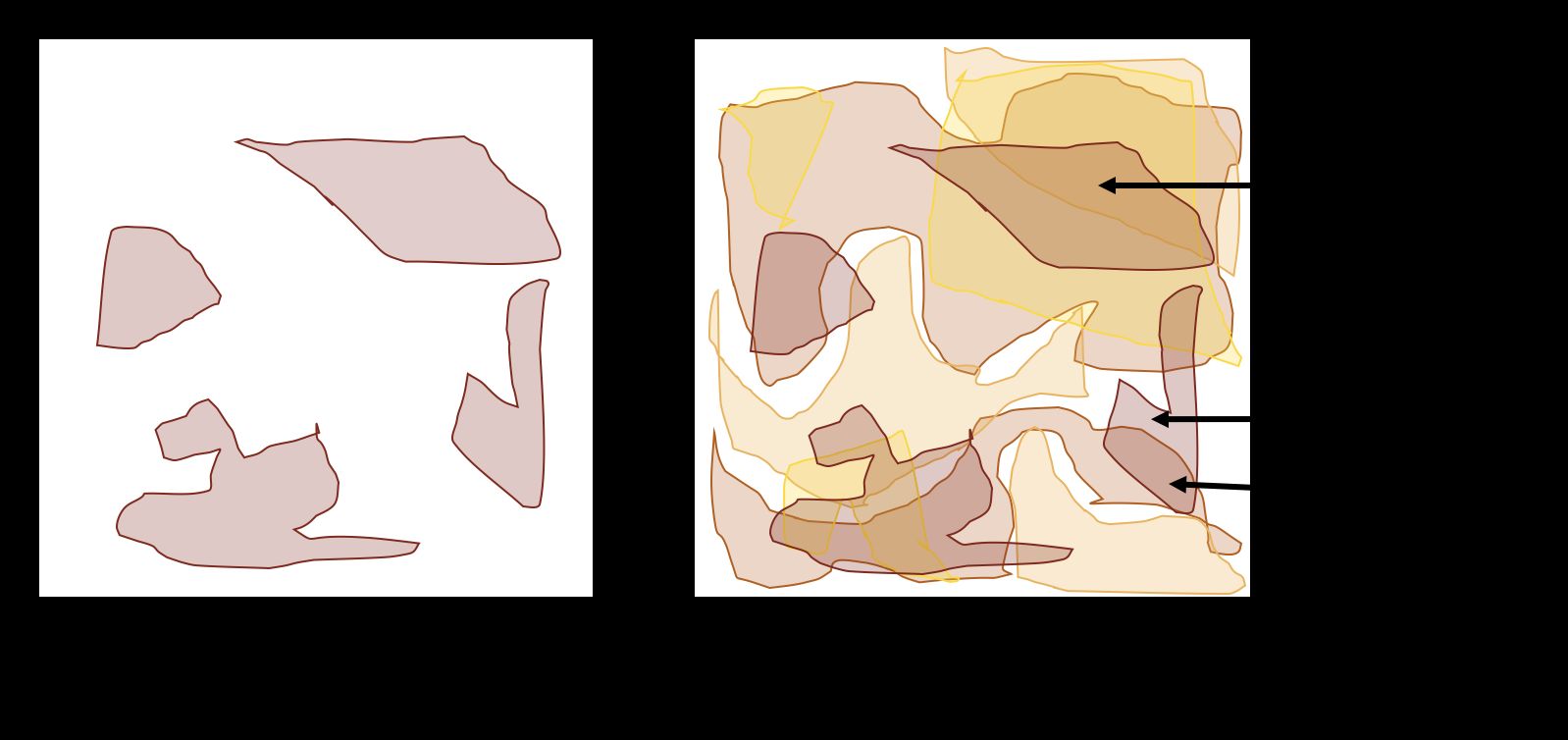
Credit: R. M. Crandall, UF/IFAS
Fire heterogeneity, or the arrangement, size, and structure of unburned patches within or around a burned area, affects the distribution of available resources. When a fire burns an animal’s home range completely, the area might become less suitable for some predators and competing species. Completely burned areas can be barriers to the movement of animals from outside the burned area, such as predators or competitors, because there is no shelter. For example, reptiles and amphibians sensitive to temperature and moisture extremes are unlikely to move across large, open, burned landscapes without shelter. Patchy fires, however, can allow animals with small home ranges to access more diverse resources. If an animal’s home range includes burned and unburned patches, the animal might search for food in unburned areas and use the burned areas for shelter because it can more readily see approaching predators (Embar et al. 2011). White-tailed deer exhibited increased movement when their home ranges included burned areas, presumably to avoid predators, relative to deer whose home ranges did not include burned areas (Cherry et al. 2018). Thus, deer still increased their use of burned areas for foraging while maintaining use of unburned areas (Cherry et al. 2018). Bees that nest in plant twigs and cavities can use patches of unburned vegetation within burned areas, but some bees can travel several kilometers to find resources (Mitchell et al. 2022).
Patchy or small burns also increase edge habitat, which is the area between two different habitats (Figure 2). Many organisms are drawn to edges because they can use each habitat to meet different needs (Wiens 1976; Harris 1988). However, the amount of edge and area within a burned or unburned patch affects habitat use differently (Sullivan et al. 2020) and can facilitate predation on some organisms. Habitat use can therefore be influenced by tradeoffs, such as the one between needing food and risking attack by predators (Cherry et al. 2017). Barriers such as busy roads and rivers that prevent plant or animal movement to different locations also affect the area that plant and animal populations require.
For plants, patchy fires provide spaces for seeds to germinate or for plants to regrow from underground organs. Patchy fires are also good for species that need unburned areas to grow larger before they burn. For example, seeds of longleaf pine (Pinus palustris) need bare ground, or burned areas, in which to germinate. Early growth occurs mainly in unburned patches, which allow young trees to be sheltered from fires until they reach a larger size (Robertson et al. 2019). Fire patchiness can interact with the wetness and dryness of landscapes to provide habitat for different plant species. In north Florida pine savannas, for example, dry upland areas that burn completely provide habitat for resprouting Hypericum microsepalum. In contrast, two other reseeding Hypericum species grow in wetter lowland areas that burn infrequently and patchily (Crandall and Platt 2012). Thus, patchy or heterogeneous fires allow species with different life histories to grow in the same burn area.
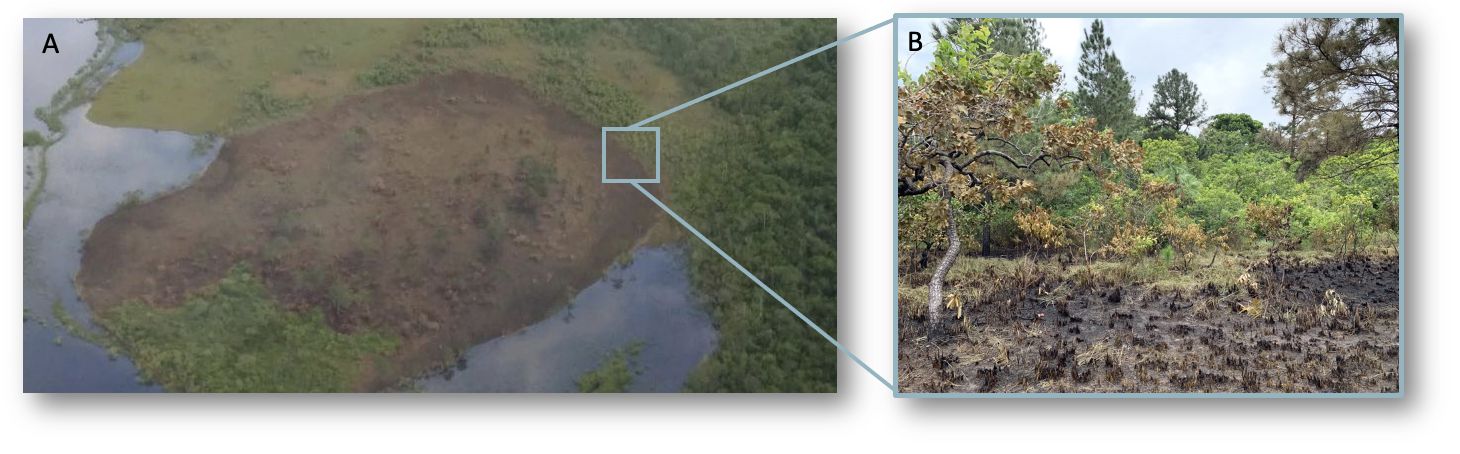
Credit: R. M. Crandall, UF/IFAS
Variation over Time
Immediately after a fire, there are winners and losers. For example, predators such as snakes and raptors tend to be more successful immediately after fires than smaller mammals such as raccoons, bobcats, and coyotes which avoid recently burned areas (Conner et al. 2011). This can make recently burned areas dangerous for smaller organisms (e.g., mice, lizards, etc.) but safer for larger organisms such as deer (Conner et al. 2011). In general, immediately after a fire, there is less food in the burned area for most animals (Lashley et al. 2011; Lashley et al. 2015); this is especially true if the burn is complete or not very patchy.
Over time, vegetation regrows in burned areas, and animal habitat use changes. For example, wild turkey and white-tailed deer increase their use of recently burned areas over time (Main and Richardson 2002), and more habitat becomes available for bee species as the vegetation returns and begins to flower (Mitchell et al. 2022). Regrowing vegetation can be useful for an animal up to a point; for instance, deer have been shown to use 4-year-old vegetation patches less than more recently burned patches (Main and Richardson 2002). The effects of fire over time also depend on the animal’s lifespan. For example, if an organism lives for only a few days, vegetation in completely burned areas might not regrow sufficiently to provide necessary resources, and the individual will need unburned areas to survive as long as possible. Home ranges can differ between seasons (Niedzielski and Bowman 2016); therefore, fire seasonality may influence habitat availability in one season but not in another.
For some plant species, reproduction only or primarily occurs in the immediate aftermath of a fire. For instance, many plants in the sand pine ecosystem and other scrubby ecosystems in Florida, such as rosemary scrub, are favored by large areas of bare ground after an intense fire that kills all the adult plants (Greenberg 2003). Reproduction happens within a short post-fire window, and the vegetation recovers over time. This is why fire is so important for many fire-dependent plant species.
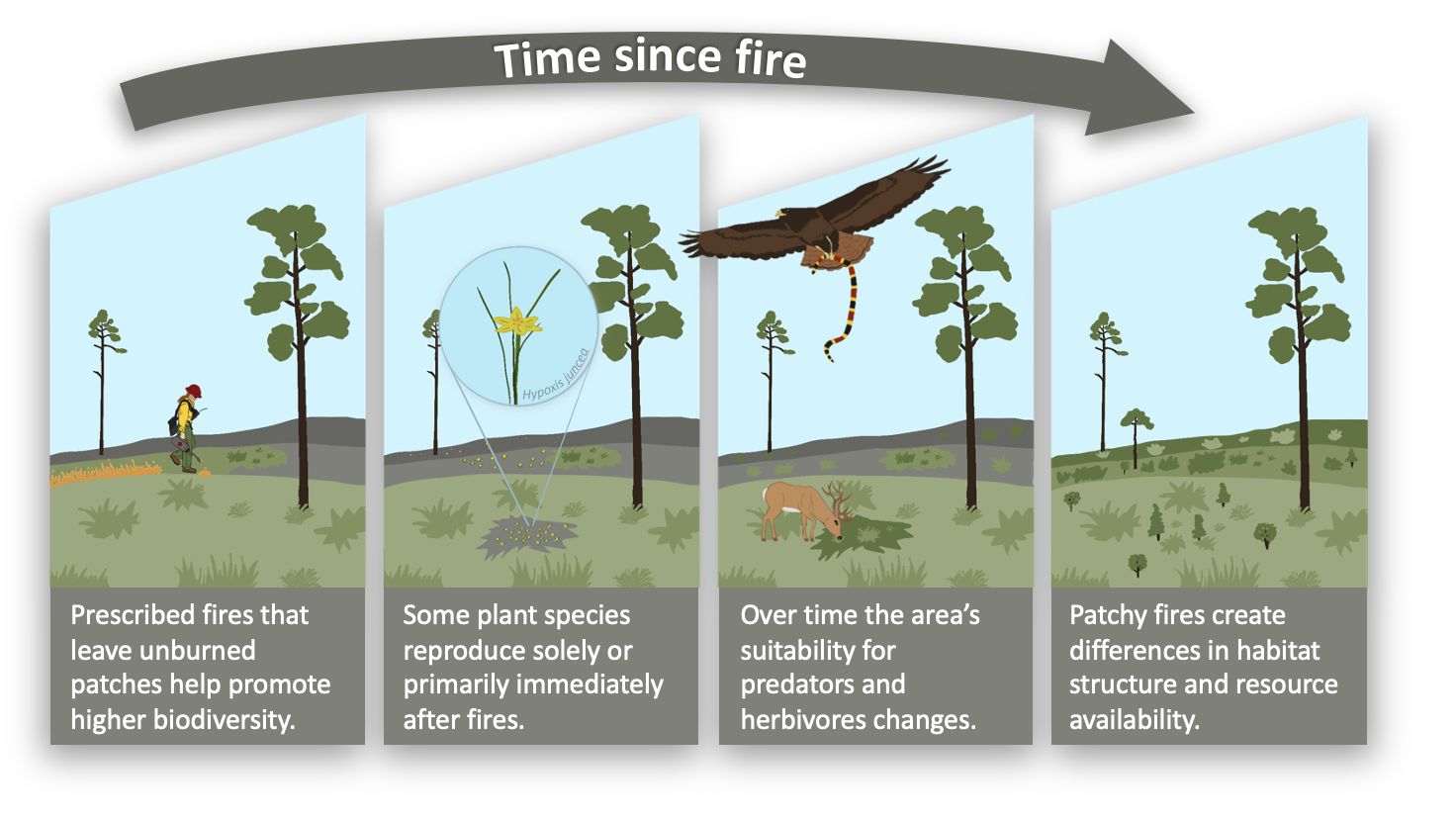
Credit: R. M. Crandall, UF/IFAS
Management Considerations
Management goals can include providing habitat for specific species or increasing overall pyrodiversity. To achieve a consistent or variable fire regime, managers can adjust the area burned in prescribed fires, the weather conditions under which they burn, and the time since the previous fire. More variable fire regimes are generally considered to provide the greatest pyrodiversity value.
The most appropriate acreage to burn in prescribed fire regimes depends on management goals. When developing management objectives targeting specific plants or animals, consider animal home range size and habitat needs, and plant seed dispersal and reproduction. For example, research suggests that burn blocks of 40–60 acres, with 50% of them burned annually, support nesting areas and food for northern bobwhite quail (Palmer and Sisson 2017; Rosche et al. 2019). Studies recommend burning 12%–33% of burn blocks (Wann et al. 2020) that are between 50 and 500 acres (Chamberlain 2019; Sullivan et al. 2020) to support habitat needs when managing wild turkeys. If management objectives include supporting a broader variety of species, consider changing the size of a fire in a given area for each burn.
For a given fire size, patchy or more heterogeneous fires tend to maximize biodiversity. Fires that leave at least some unburned patches can help support a greater variety of species, including those that benefit from recent or past burns or both in close proximity (Figure 3). Management strategies to maximize biodiversity might include patch-mosaic burning. Patch-mosaic burning means creating patchy fires by burning in less favorable fire conditions (e.g., high relative humidity), using less intense firing techniques (e.g., back-burning or widely spaced point source) (Waldrop and Goodrick 2012), and not going back to ignite patches that failed to burn. All of these techniques should increase burn patchiness in space and time. This can also be accomplished by increasing the overall fire size and letting fires burn in a natural pattern along gradients of fuel and soil moisture. If the goal is to manage for specific species, managers can identify the area an animal needs for particular resources. With that information, managers can then decide which areas to burn completely and which to burn heterogeneously.
Below are a few useful definitions for understanding the effects of fire over space and time.
Natural range of variability: Historical amount of variation in fire regime and ecosystem characteristics. In the United States, this is considered prior to European settlement (Jones and Tingley 2021).
Patchiness: Variation in size and arrangement of vegetation patches or burned areas on a landscape (Jones and Tingley 2021).
Patch-mosaic burning: Establishing a network or mosaic of vegetation patches that have different structures to maximize biodiversity. Burns are ignited in different areas across the landscape and allowed to burn naturally (Jones and Tingley 2021).
Pyrodiversity: Biodiversity that results from the interactions between fire regimes and animal and plant biodiversity, especially related to food webs (Bowman et al. 2016).
Table 1. A list of fire characteristics, definitions, and examples of how the variability of each characteristic creates a fire regime for select plants and animals.
Acknowledgments
The authors acknowledge funding for the fire science delivery program, “The Southern Fire Exchange: Putting Fire Science on the Ground,” from the Joint Fire Science Program and in agreement with the United States Forest Service, Southern Research Station. The Southern Fire Exchange supports the dissemination of and access to fire science information. To learn more, visit https://southernfireexchange.org/.
References
Baruzzi, C., and R. M. Crandall. 2021. “JFSP-funded research informed fire management to improve habitat for the endangered Florida bonneted bat.” SFE Success Story 2021—October.
Bowman, D. M., G. L. Perry, S. I. Higgins, C. N. Johnson, S. D. Fuhlendorf, and B. P. Murphy. 2016. “Pyrodiversity is the coupling of biodiversity and fire regimes in food webs.” Philosophical Transactions of the Royal Society B: Biological Sciences 371:20150169. https://doi.org/10.1098/rstb.2015.0169
Braun de Torrez, E. C., H. K. Ober, and R. A. McCleery. 2018. “Activity of an endangered bat increases immediately following prescribed fire.” The Journal of Wildlife Management 82:1115–1123. https://doi.org/10.1002/jwmg.21481
Chamberlain, M. 2019. “Southern Fire Exchange Webinar: Influence of Prescribed Fire on the Ecology of Wild Turkeys.” https://www.youtube.com/watch?v=RueQUgPN-ZM
Cherry, M. J., R. J. Warren, and L. M. Conner. 2017. “Fire‐Mediated Foraging Tradeoffs in White‐Tailed Deer.” Ecosphere 8:e01784. https://doi.org/10.1002/ecs2.1784
Cherry, M. J., R. B. Chandler, E. P. Garrison, D. A. Crawford, B. D. Kelly, D. B. Shindle, K. G. Godsea, K. V. Miller, and L. M. Conner. 2018. “Wildfire affects space use and movement of white-tailed deer in a tropical pyric landscape.” Forest Ecology and Management 409:161–169.
Conner, L. M., S. B. Castleberry, and A. M. Derrick. 2011. “Effects of Mesopredators and Prescribed Fire on Hispid Cotton Rat Survival and Cause-Specific Mortality.” The Journal of Wildlife Management 75:938–944. https://doi.org/10.1002/jwmg.110
Crandall, R. M. 2011. “The Ecology of Congeneric Resprouters and Reseeders (Hypericum spp.) along Fire-Frequented Pine Savanna Ecoclines.” LSU Digital Commons. https://digitalcommons.lsu.edu/gradschool_dissertations/150/
Crandall, R. M., and W. J. Platt. 2012. “Habitat and fire heterogeneity explain the co-occurrence of congeneric resprouter and reseeder Hypericum spp. along a Florida pine savanna ecocline.” Plant Ecology 213:1643–1654. https://doi.org/10.1007/s11258-012-0119-0
Embar, K., B. P. Kotler, and S. Mukherjee. 2011. “Risk Management in Optimal Foragers: The Effect of Sightlines and Predator Type on Patch Use, Time Allocation, and Vigilance in Gerbils.” Oikos 120:1657–1666. https://doi.org/10.1111/j.1600-0706.2011.19278.x
Fill, J. M., and R. M. Crandall. 2018. “Quail, Turkey, and Deer: Fire Effects and Management Recommendations.” Southern Fire Exchange Factsheet 2018-8. https://southernfireexchange.org/wp-content/uploads/2018-8.pdf
Greenberg, C. H. 2003. “Vegetation Recovery and Stand Structure following a Prescribed Stand-Replacement Burn in Sand Pine Scrub.” Natural Areas Journal 23:141–151.
Harris, L. D. 1988. “Edge Effects and Conservation of Biotic Diversity.” Conservation Biology 2:330–332. https://doi.org/10.1111/j.1523-1739.1988.tb00196.x
Jones, G. M., and M. W. Tingley. 2022. “Pyrodiversity and Biodiversity: A History, Synthesis, and Outlook.” Diversity and Distributions 28:386–403. https://doi.org/10.1111/ddi.13280
Kamps, J. T., W. E. Palmer, T. M. Terhune, G. Hagan, and J. A. Martin. 2017. “Effects of Fire Management on Northern Bobwhite Brood Ecology.” European Journal of Wildlife Research 63:1–10. https://doi.org/10.1007/s10344-017-1078-5
Knapp, B. O., L. S. Pile, J. L. Walker, and G. Wang. 2018. “Fire Effects on a Fire-Adapted Species: Response of Grass Stage Longleaf Pine Seedlings to Experimental Burning.” Fire Ecology 14:1–16. https://doi.org/10.1186/s42408-018-0003-y
Knight, T. M., and R. D. Holt. 2005. “Fire generates spatial gradients in herbivory: An example from a Florida sandhill ecosystem.” Ecology 86:587–593. https://doi.org/10.1890/04-1069
Lashley, M. A., C. A. Harper, G. E. Bates, and P. D. Keyser. 2011. “Forage Availability for White-Tailed Deer following Silvicultural Treatments in Hardwood Forests.” The Journal of Wildlife Management 75:1467–1476. https://doi.org/10.1002/jwmg.176
Lashley, M. A., M. C. Chitwood, C. A. Harper, C. S. DePerno, and C. E. Moorman. 2015. “Variability in Fire Prescriptions to Promote Wildlife Foods in the Longleaf Pine Ecosystem.” Fire Ecology 11:62–79. https://doi.org/10.4996/fireecology.1103062
Main, M. B., and L. W. Richardson. 2002. “Response of Wildlife to Fire in Southwest Florida Pine Flatwoods.” Wildlife Society Bulletin 30:213–221.
Martin, R. E., and D. B. Sapsis. 1992. “Fires as agents of biodiversity: Pyrodiversity promotes biodiversity.” In Proceedings: Symposium on Biodiversity of Northwestern California, edited by H. M. Kerner. 150–157. Center of Wildland Resources Report (No. 29).
Meyer, R. T., S. M. Pokswinski, J. Ney, and D. McElveen. 2023. “Pupae Survival following Fire in the Frosted Elfin (Callophrys irus).” Agricultural and Forest Entomology 25:336–343.
Mitchell, N., S. A. Weaver, and R. M. Crandall. 2022. “Bees and fire: How does fire in longleaf pine savannas affect bee communities? FOR383/FR454, 6/2022.” EDIS 2022 (3). https://doi.org/10.32473/edis-fr454-2022
Niedzielski, B., and J. Bowman. 2016. “Home Range and Habitat Selection of the Female Eastern Wild Turkey at Its Northern Range Edge.” Wildlife Biology 22:55–63. https://doi.org/10.2981/wlb.00138
Palmer, W. E., and D. C. Sisson. 2017. Tall Timbers' Bobwhite Quail Management Handbook. Tall Timbers Press.
Powell, R. A., and M. S. Mitchell. 2012. “What is a home range?” Journal of Mammalogy 93:948–958. https://doi.org/10.1644/11-MAMM-S-177.1
Robertson, K. M., W. J. Platt, and C. E. Faires. 2019. “Patchy fires promote regeneration of longleaf pine (Pinus palustris Mill.) in pine savannas.” Forests 10:367. https://doi.org/10.3390/f10050367
Rosche, S. B., C. E. Moorman, K. Pacifici, J. G. Jones, and C. S. DePerno. 2019. “Northern Bobwhite Breeding Season Habitat Selection in Fire-Maintained Pine Woodland.” The Journal of Wildlife Management 83:1226–1236. https://doi.org/10.1002/jwmg.21683
Spier, L. P., and J. R. Snyder. 1998. “Effects of Wet- and Dry-Season Fires on Jacquemontia curtissii, a South Florida Pine Forest Endemic.” Natural Areas Journal 18:350–357.
Steel, Z. L., B. M. Collins, D. B. Sapsis, and S. L. Stephens. 2021. “Quantifying Pyrodiversity and Its Drivers.” Proceedings of the Royal Society B 288:20203202. https://doi.org/10.1098/rspb.2020.3202
Sullivan, D. J., K. D. McEntire, B. S. Cohen, B. A. Collier, and M. J. Chamberlain. 2020. “Spatial scale and shape of prescribed fires influence use by wild turkeys.” The Journal of Wildlife Management 84:1570–1577. https://doi.org/10.1002/jwmg.21944
Waldrop, T. A., and S. L. Goodrick. 2012. Introduction to Prescribed Fire in Southern Ecosystems. Science Update SRS-054. Asheville, NC: United States Department of Agriculture Forest Service, Southern Research Station.
Wann, G. T., J. A. Martin, and M. J. Chamberlain. 2020. “The Influence of Prescribed Fire on Wild Turkeys in the Southeastern United States: A Review and Synthesis.” Forest Ecology and Management 455:117661. https://doi.org/10.1016/j.foreco.2019.117661
Wiens, J. A. 1976. “Population Responses to Patchy Environments.” Annual Review of Ecology and Systematics 7:81–120. https://doi.org/10.1146/annurev.es.07.110176.000501



