Northern House Mosquito Culex pipiens Linnaeus, 1758 (Insecta: Diptera: Culicidae)
The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The northern house mosquito, Culex pipiens Linnaeus, is considered as a major vector of pathogens worldwide. In North America, this mosquito is widely distributed in urban, suburban, and rural areas. Culex pipiens has the ability to transmit pathogens, such as West Nile virus (WNV) and Saint Louis encephalitis virus (SLEV) (Reeves et al. 1942, Dohm et al. 2002, Molaei et al. 2006, Turell 2012). Culex pipiens is also responsible for transmitting other pathogens, including Rift Valley fever virus (RVFV), Sindbis virus (SINV), and roundworms that cause filariasis in many regions of the globe (Taylor et al. 1955, Meegan et al. 1980, Michalski et al. 2010).
Synonymy
Culex consobrinus Robineau-desvoidy, 1827 (ITIS 2020).
Distribution
Culex pipiens is native to Africa but has been introduced to many regions throughout the world (Figure 1) (Ross 1964, Harbach 2012). In North America, Culex pipiens, is found in northern United States and southern Canada in areas above 39° north latitude, whereas the closely related Culex quinquefasciatus, known as the southern house mosquito, is found at latitudes below 36° north (Figure 1). Between 32° and 40° north latitudes, there is a broad hybridization zone, in which Culex pipiens, Culex quinquefasciatus, and their hybrids exist (Figure 1) (Kothera et al. 2009, Huang et al. 2011). Mating between these two species within the hybrid zone can generate viable hybrid progeny. In addition to being found in North America, Culex pipiens is broadly distributed in many other regions, including South America, the Middle East, Asia, Africa, and Europe (Figure 1) (Vinogradova 2000).
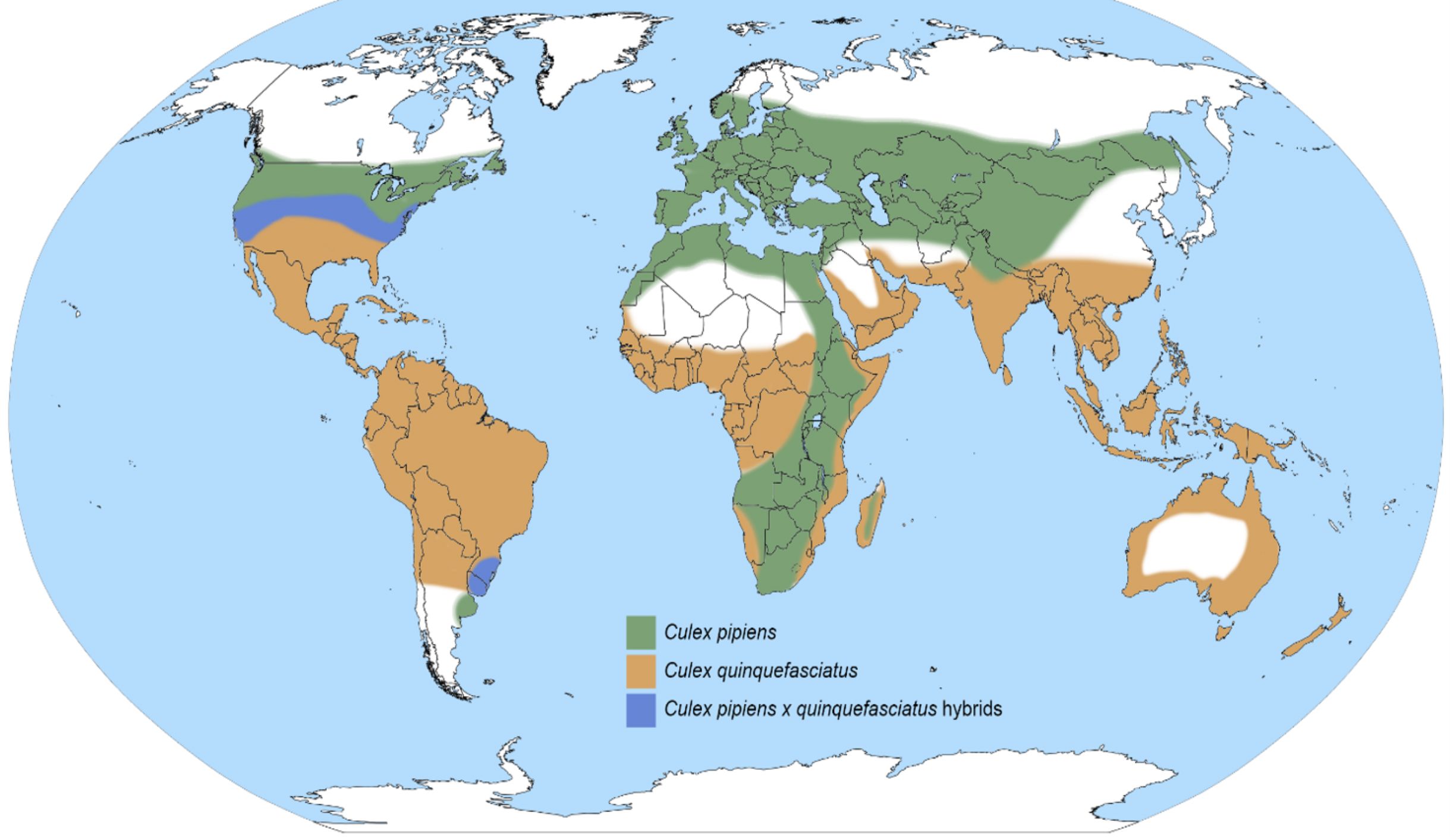
Credit: Map redrawn after Ciota and Kramer 2013 by Nathan D. Burkett-Cadena, University of Florida
Description
Egg
The eggs of Culex pipiens are laid together in the shape of an oval raft on the surface of stagnant water (Figure 2). Egg rafts typically contain 150-300 eggs. The raft remains floating on the water surface until larval emergence.

Credit: Lawrence E. Reeves, University of Florida
Larva
Culex pipiens larvae are small in size and pale to brown in color (Figure 3-A). The larval head is wider than long (Figure 3-B). The head bears the mouthparts, a pair of compound eyes and antennae (Foster and Walker 2002). Larval mouthparts are composed of lateral brushes, which filter the organic materials from the water (Foster and Walker 2002). Larval antennae are shorter than the head in length (Figure 3-B). The abdomen is cylindrical and composed of eight apparent segments. Abdominal setae 6 on segments III and IV are usually double (Figure 3-C) (Carpenter and La Casse 1974). The long and thin siphon arises from the eighth abdominal segment (Darsie and Ward 2005). The siphon of the larva is usually four to five times longer than its width at the midpoint (Figure 3-D) (Burkett-Cadena 2013). Segment VIII contains a patch of comb scales, and the saddle, which encircles the tenth abdominal segment, is complete and darker from a dorsal view (Darsie and Ward 2005).
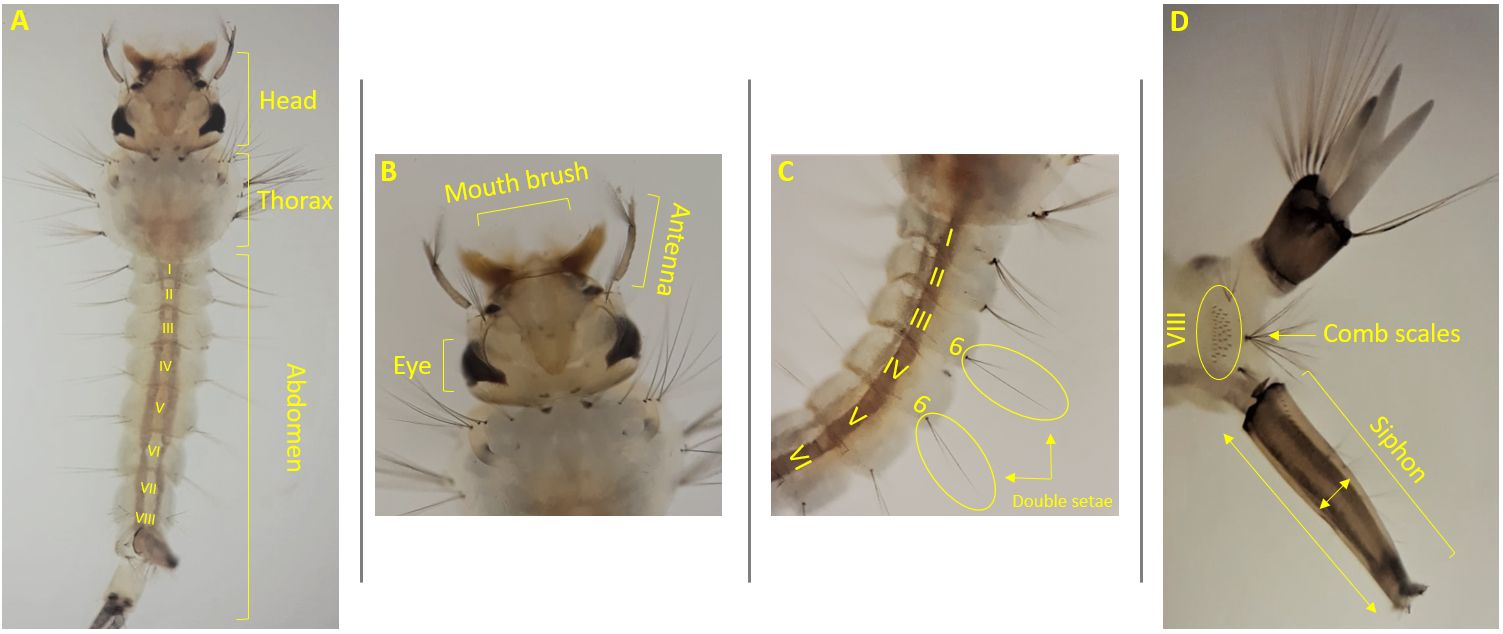
Credit: Abdullah A. Alomar, University of Florida
Pupa
Like all mosquito pupae, Culex pipiens pupae have a comma-like shape. The pupal head and thorax are fused in a cephalothorax, and the abdomen is curled beneath it (Figure 4-A) (Foster and Walker 2002). The pupa is a non-feeding stage, but it has a pair of respiratory trumpets located dorsally on the cephalothorax (Figure 4-B). These trumpets allow pupae to obtain oxygen at the surface of water. The pupal abdomen contains eight segments as well as a pair of large paddles at the apex of the abdomen (Foster and Walker 2002). The two broad paddles are used for swimming (Figure 4-A). Pupae become darker in color prior to adult emergence.
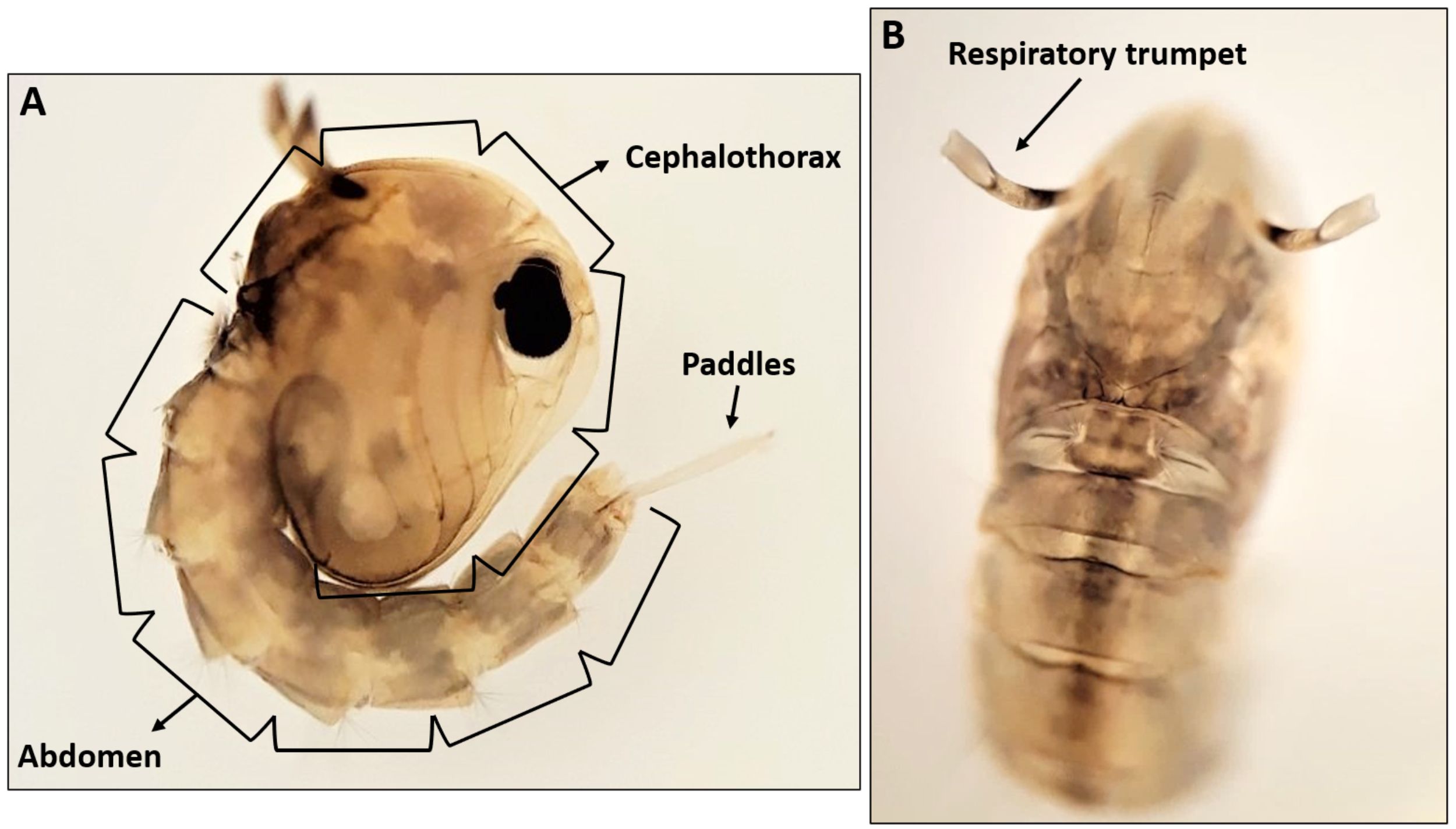
Credit: Abdullah A. Alomar, University of Florida
Adult
An adult Culex pipiens is a small to medium-sized mosquito. The adult body consists of the head (eyes, antennae, proboscis and palps), thorax (legs and wings), and abdomen (ten segments and genitalia). Generally, the body of Culex pipiens tends to be light brown with scattered patches of pale scales (Figure 5). The proboscis is dark in color. Both proboscis and antennae have roughly the same length. The scutum (dorsal portion of thorax) is brown and is covered in with narrow golden-brown scales (Carpenter and La Casse 1974). Each abdominal segment has basal pale bands which are constricted at two points dorsolaterally. The apex of the abdomen is obviously rounded when it is viewed dorsally (Darsie and Ward 2005). Adult wings are 3.5 to 4.0 mm in length with narrow dark scales (Carpenter and La Casse 1974).

Credit: Lawrence E. Reeves, University of Florida
Life Cycle
Like all mosquito species, Culex pipiens undergoes complete metamorphosis, passing through four distinct stages: egg, larva, pupa, and adult (Figure 6). The life cycle begins with the egg, where gravid females of Culex pipiens lay their eggs in rafts of 150-300 eggs on the surface of standing water (Wood et al. 1979, Vinogradova 2000). Culex pipiens larvae can tolerate water with high levels of organic content and are found in a wide range of larval habitats, including ponds, water-filled containers (e.g., buckets, rain barrels, cans), roadside ditches, and sewage seepage (Foster and Walker 2002, Wood et al. 1979). Eggs hatch within 2-3 days into small larvae that emerge from the lower end of the eggs. Each larva undergoes four stages of growth known as instars (Foster and Walker 2002). Larvae feed on organic matter, microscopic organisms, animal and plant particles in their aquatic habitats (Foster and Walker 2002). Larvae have the ability to move up and down the water column to obtain food and oxygen. At the end of the fourth instar (i.e., the final instar), larvae molt into pupae. Pupae remain at the water surface most of the time to breathe air through their trumpets. After 2 to 3 days of development in the pupal stage, adults (i.e., imago) emerge from the pupal case and gain the ability to fly shortly thereafter. Both adult males and females need sugar as a source of carbohydrate for energy, which is obtained from flower nectar, extrafloral nectar, damaged fruit, or homopteran honeydew (Foster 1995). However, only females require a blood meal as a source of protein for developing eggs (Foster and Walker 2002). Female Culex pipiens obtain blood meals primarily from birds and/or mammals, including humans. During the winter, Culex pipiens may overwinter as a fertilized adult female in sheltered places, such as caves and basements (Wood et al. 1979). In the spring, adult females emerge and seek a blood meal to develop a batch of eggs (Wood et al. 1979).
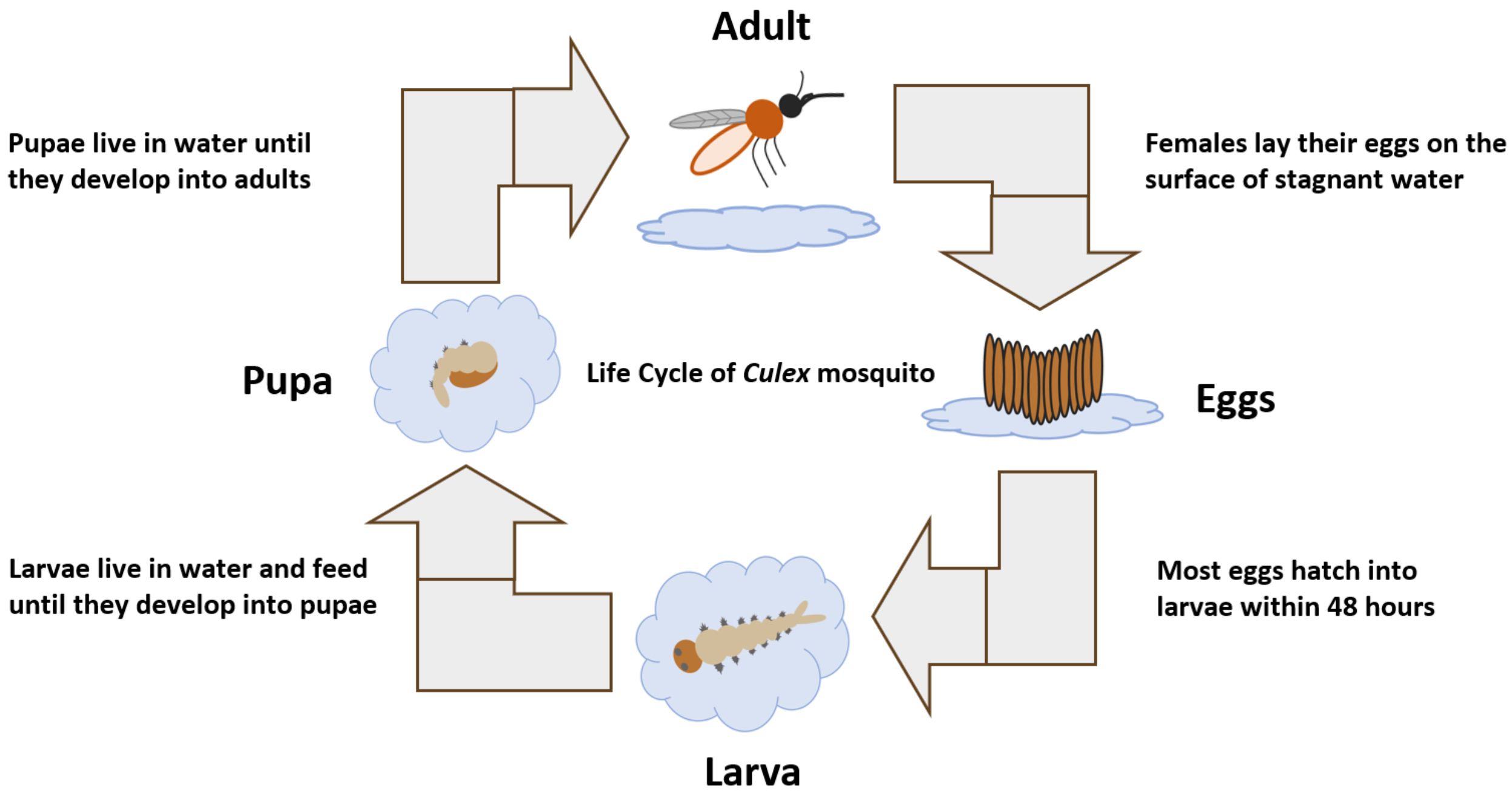
Credit: Abdullah A. Alomar, University of Florida
Medical Importance
The northern house mosquito Culex pipiens plays a critical role in the transmission of pathogens to wildlife, livestock, and humans. Pathogens transmitted by this species include viruses (e.g., WNV, RVFV, SLEV, SINV), filarial worms (e.g., canine heartworm, human filarial nematodes), and protozoa (e.g., protozoans that cause avian malaria) (Reisen et al. 1992, Bøgh et al. 1998, Turell et al. 2002, Kimura et al. 2010, Ledesma and Harrington 2011).
West Nile virus is an emerging zoonotic mosquito-borne pathogen that can cause life-threatening disease in humans and animals. In the northern United States, Culex pipiens is considered the primary vector for WNV transmission (Turell et al. 2002, Kilpatrick et al. 2005, Anderson and Main 2006, Kramer et al. 2008). Studies of host-feeding patterns of Culex pipiens showed that birds are the primary blood meal hosts for this species and also serve as the primary amplification host for WNV, which indicates an important contribution of birds to WNV epidemiology in the northern United States (Hamer et al. 2008, Hamer et al. 2009). Several bird species, including the American robin (Turdus migratorius), house sparrow (Passer domesticus), and blue jay (Cyanocitta cristata), have been implicated as important hosts (i.e., competent reservoir hosts) for WNV (Komar et al. 2003, Hamer et al. 2009, Duggal et al. 2014). Mammals, such as horses and humans are regarded as dead-end hosts which means they can acquire WNV via the bite of an infected mosquito, but their blood viremia levels never reach high enough levels to allow transmission to mosquitoes (Figure 7) (Murray et al. 2010).
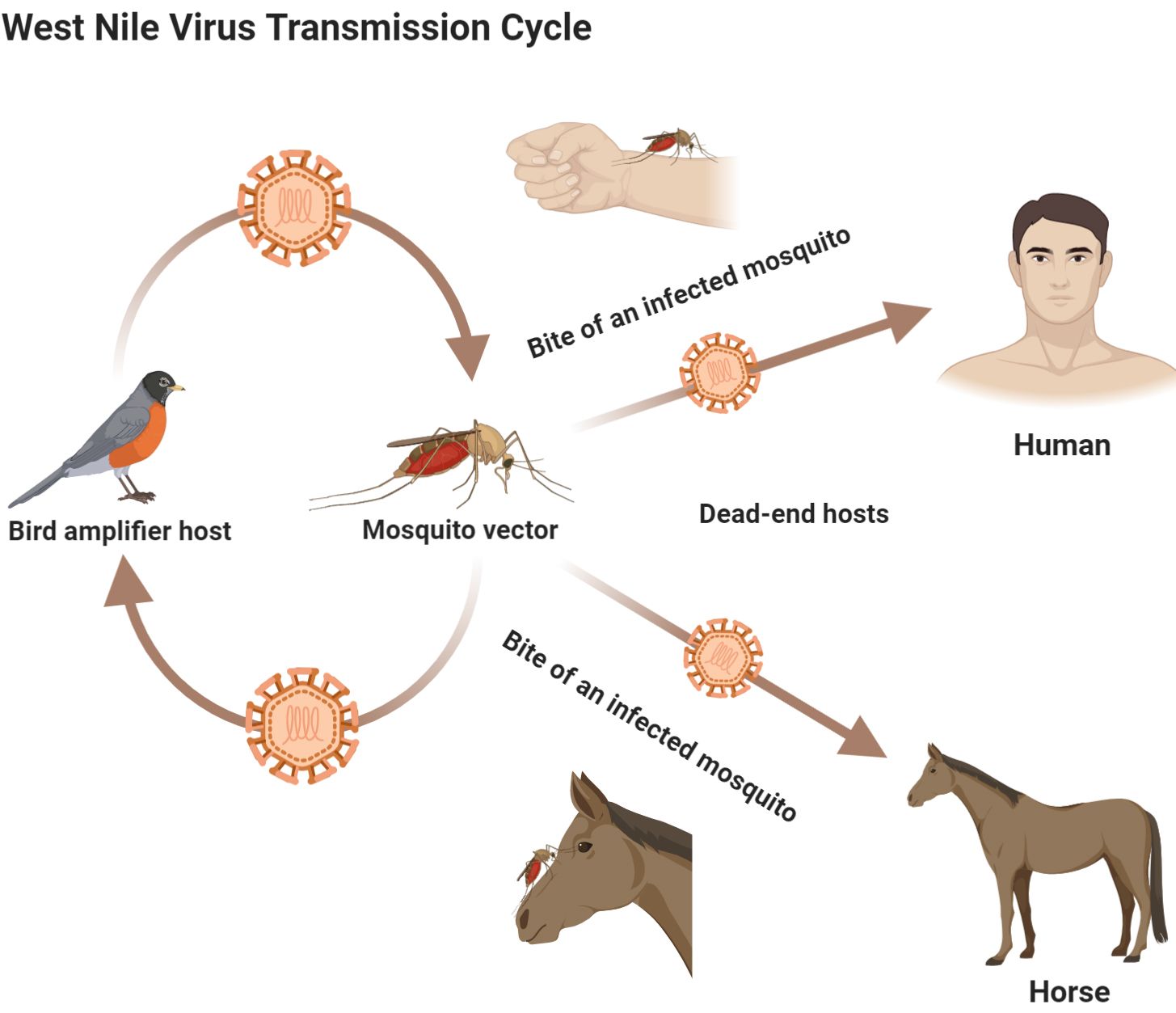
Credit: Abdullah A. Alomar, University of Florida
Humans infected with WNV may experience no symptoms (asymptomatic), mild symptoms, such as body aches, vomiting, headache, joint pains, rash, or diarrhea (CDC 2018), or in some cases, severe illness (e.g., encephalitis or meningitis) that may even lead to death (Murray et al. 2006). In the United States, 958 cases of WNV infection in humans were reported in 2019, and more than 50% of these cases had severe illness (CDC 2020). The chance of developing serious neuroinvasive disease (febrile illness with inflammation of the central nervous system) increases with age (Reimann et al. 2008, CDC 2018).
Outside the United States, particularly in Africa and the Arabian Peninsula, Culex pipiens has been incriminated as a vector for Rift Valley fever virus (RVFV). RVFV is an acute zoonotic infectious disease that mainly affects livestock but can also affect humans. Sheep, cattle, and goats are considered important livestock that serve as amplification hosts for RVFV (Linthicum et al. 2016). Humans can acquire RVFV through the bite of an infected mosquito or through direct contact with blood, fluids, or tissues from infected livestock (Figure 8) (CDC 2018). Human infection with RVFV can cause severe illness, including eye disease, encephalitis/meningoencephalitis, and/or hemorrhagic fever that can be fatal. The majority of people infected with RVFV, however, show either no symptoms or mild symptoms, including back pain, fever, weakness, and dizziness (CDC 2018).
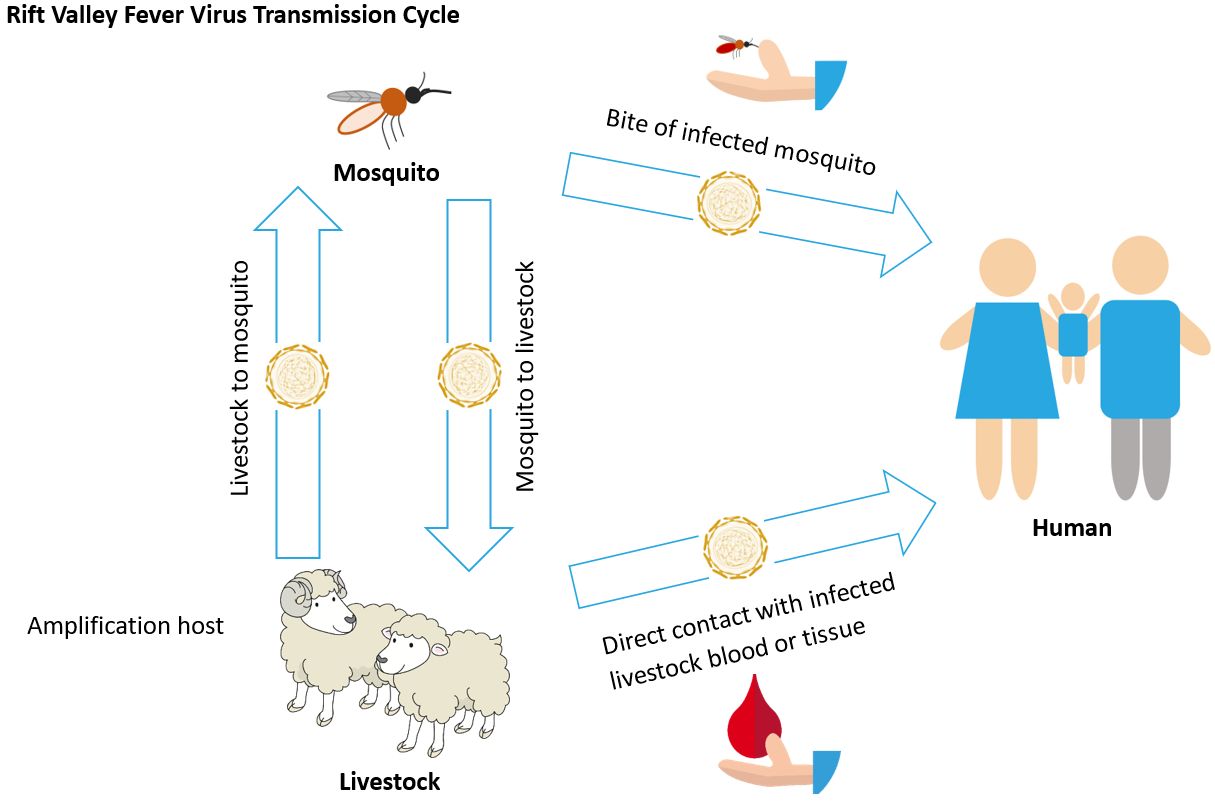
Credit: Abdullah A. Alomar, University of Florida
Culex pipiens is also known as a vector of the filarial nematode Wuchereria bancrofti, a parasitic worm-like nematode that infects the lymphatic system causing lymphatic filariasis in humans. When eggs from adult W. bancrofti hatch in an infected host, microscopic worms (called microfilariae) enter peripheral circulation (i.e., blood vessels that circulate blood to the extremities) where they can be ingested by female Culex pipiens during blood feeding process. Once consumed in a blood meal, the microfilariae penetrate the midgut wall of the mosquito, develop into third stage infective larvae inside the female mosquito’s flight muscles, and then migrate to the mouthparts before being transmitted to humans during biting (Foster and Walker 2002). There are millions of people living under the risk of lymphatic filariasis in tropical and subtropical regions of Africa, the Western Pacific, South America, and Asia (Bockarie et al. 2009). The World Health Organizations has identified lymphatic filariasis as a leading cause of permanent disability globally (WHO 2020). When left untreated, humans infected with W. bancrofti can develop lymphedema, elephantiasis, and hydrocele (swelling of the scrotum in males). These symptoms develop in most people after years of being infected (CDC 2018).
Management
Successful control of Culex pipiens requires integrated mosquito management, an approach that targets the mosquito at multiple life stages through a variety of control tools (Rose 2001). For more information on managing mosquitoes, please visit the EDIS document on integrated mosquito management at https://edis.ifas.ufl.edu/publication/IN1045. The pdf is available at https://edis.ifas.ufl.edu/pdf/IN/IN104500.pdf.
Selected References
Anderson JF, Main AJ. 2006. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the northeastern United States. The Journal of Infectious Diseases 194(11):1577-9.
Bøgh C, Pedersen EM, Mukoko DA, Ouma JH. 1998. Permethrin-impregnated bednet effects on resting and feeding behaviour of lymphatic filariasis vector mosquitoes in Kenya. Medical and Veterinary Entomology 12(1):52-9.
Burkett-Cadena ND. 2013. Mosquitoes of the Southeastern United States. University of Alabama Press, Tuscaloosa, Alabama. 64p.
Bockarie MJ, Pedersen EM, White GB, Michael E. 2009. Role of vector control in the global program to eliminate lymphatic filariasis. Annual review of entomology 54:469-87.
Carpenter SJ, La Casse WJ. 1974. Mosquitoes of North America (North of Mexico). University of California Press, Berkeley, California. 284-285pp.
CDC. (2018). Diseases. Center for Disease Control. https://www.cdc.gov/diseasesconditions/index.html (9 July 2020).
CDC. (2020). West Nile virus. Center for Disease Control. https://www.cdc.gov/westnile/statsmaps/preliminarymapsdata2019/index.html (9 July 2020).
Ciota AT, Kramer LD. 2013. Vector-virus interactions and transmission dynamics of West Nile virus. Viruses 5(12):3021-47.
Dohm DJ, Sardelis MR, Turell MJ. 2002. Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera: Culicidae). Journal of Medical Entomology 39(4):640-4.
Darsie Jr RF, Ward RA. 2005. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. University Press of Florida, Gainesville, Florida. 79-115pp.
Duggal NK, Bosco-Lauth A, Bowen RA, Wheeler SS, Reisen WK, Felix TA, Mann BR, Romo H, Swetnam DM, Barrett AD, Brault AC. 2014. Evidence for co-evolution of West Nile virus and house sparrows in North America. PLOS Neglected Tropical Diseases 8(10):e3262.
Foster WA. 1995. Mosquito sugar feeding and reproductive energetics. Annual review of entomology 40(1):443-74.
Foster WA, Walker ED. 2002. Mosquitos (Culicidae). In: Mullen G, Durden L, editors. Medical and Veterinary Entomology. Academic Press, San Diego, California. 261-317pp.
Hamer GL, Walker ED, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Schotthoefer AM, Brown WM, Wheeler E, Kitron UD. 2008. Rapid amplification of West Nile virus: The role of hatch-year birds. Vector-Borne and Zoonotic Diseases 8(1):57-68.
Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. 2009. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. The American Journal of Tropical Medicine and Hygiene 80(2):268-78.
Huang S, Molaei G, Andreadis TG. 2011. Reexamination of Culex pipiens hybridization zone in the eastern United States by ribosomal DNA-based single nucleotide polymorphism markers. The American Journal of Tropical Medicine and Hygiene 85(3):434-41.
ITIS. (2020). Culex pipiens Linnaeus, 1758. Integrated Taxonomic Information System. http://www.itis.gov. (9 July 2020).
Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P. 2005. West Nile virus risk assessment and the bridge vector paradigm. Emerging Infectious Diseases 11(3):425.
Kramer LD, Styer LM, Ebel GD. 2008. A global perspective on the epidemiology of West Nile virus. The Annual Review of Entomology 53:61-81.
Kimura M, Darbro JM, Harrington LC. 2010. Avian malaria parasites share congeneric mosquito vectors. Journal of Parasitology 96(1):144-51.
Kothera L, Zimmerman EM, Richards CM, Savage HM. 2009. Microsatellite characterization of subspecies and their hybrids in Culex pipiens complex (Diptera: Culicidae) mosquitoes along a north-south transect in the Central United States. Journal of Medical Entomology 46(2):236-48.
Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerging Infectious Diseases 9(3):311.
Ledesma N, Harrington L. 2011. Mosquito vectors of dog heartworm in the United States: Vector status and factors influencing transmission efficiency. Topics in Companion Animal Medicine 26(4):178-85.
Linthicum KJ, Britch SC, Anyamba A. 2016. Rift Valley fever: An emerging mosquito-borne disease. Annual Review of Entomology 61:395-415.
Michalski ML, Erickson SM, Bartholomay LC, Christensen BM. 2010. Midgut barrier imparts selective resistance to filarial worm infection in Culex pipiens. PLOS Neglected Tropical Diseases 4(11):e875.
Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. 2006. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerging Infectious Diseases 12(3):468.
Meegan JM, Khalil GM, Hoogstraal H, Adham FK. 1980. Experimental transmission and field isolation studies implicating Culex pipiens as a vector of Rift Valley fever virus in Egypt. The American Journal of Tropical Medicine and Hygiene 29(6):1405-10.
Murray K, Baraniuk S, Resnick M, Arafat R, Kilborn C, Cain K, Shallenberger R, York TL, Martinez D, Hellums JS, Hellums D. 2006. Risk factors for encephalitis and death from West Nile virus infection. Epidemiology & Infection 134(6):1325-32.
Murray KO, Mertens E, Desprès P. 2010. West Nile virus and its emergence in the United States of America. Veterinary Research 41(6):67.
Pastula DM, Smith DE, Beckham JD, Tyler KL. 2016. Four emerging arboviral diseases in North America: Jamestown Canyon, Powassan, chikungunya, and Zika virus diseases. Journal of Neurovirology 22(3):257-60.
Reeves WC, McD Hammon W, Izumi EM. 1942. Experimental transmission of St. Louis encephalitis virus by Culex pipiens Linnaeus. Proceedings of the Society for Experimental Biology and Medicine 50(1):125-8.
Reisen WK, Milby MM, Presser SB, Hardy JL. 1992. Ecology of mosquitoes and St. Louis encephalitis virus in the Los Angeles Basin of California, 1987-1990. Journal of Medical Entomology 29(4):582-98.
Rose RI. 2001. Pesticides and public health: Integrated methods of mosquito management. Emerging Infectious Diseases 7(1):17.
Ross HH. 1964. The colonization of temperate North America by mosquitoes and man. Mosquito News 24(2).
Reimann CA, Hayes EB, DiGuiseppi C, Hoffman R, Lehman JA, Lindsey NP, Campbell GL, Fischer M. 2008. Epidemiology of neuroinvasive arboviral disease in the United States, 1999-2007. The American Journal of Tropical Medicine and Hygiene 79(6):974-9.
Taylor RM, Hurlbut HS, Work TH, Kingston JR, Frothingham TE. 1955. Sindbis virus: A newly recognized arthropod-transmitted virus. The American Journal of Tropical Medicine and Hygiene 4(5):844-62.
Turell MJ, Sardelis MR, O'Guinn ML, Dohm DJ. 2002. Potential Vectors of West Nile Virus in North America. In: Mackenzie J, Barrett A, Deubel V, editors. Japanese Encephalitis and West Nile Viruses. Volume 267 Current Topics in Microbiology and Immunology. Springer, Berlin, Heidelberg. 241-252pp.
Turell MJ. 2012. Members of the Culex pipiens complex as vectors of viruses. Journal of the American Mosquito Control Association 28(4s):123-6.
Vinogradova EB. 2000. Culex pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetics, Applied importance and Control. Pensoft Publishers, Sofia, Bulgaria. 36-58pp.
WHO. (2020). Lymphatic filariasis. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (9 July 2020).
Wood DM, Dang PT, Ellis RA. 1979. The Mosquitoes of Canada (Diptera: Culicidae) Part 6. The Insects and Arachnids of Canada. Research Branch. Canada Department of Agriculture Publication, Ottawa, Ontario. 274-276pp.



