The Lychee Erinose Mite Aceria litchii (Keifer) (Acari: Eriophyidae)
Introduction
The purpose of this publication is to provide an in-depth profile of the lychee erinose mite. It is intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
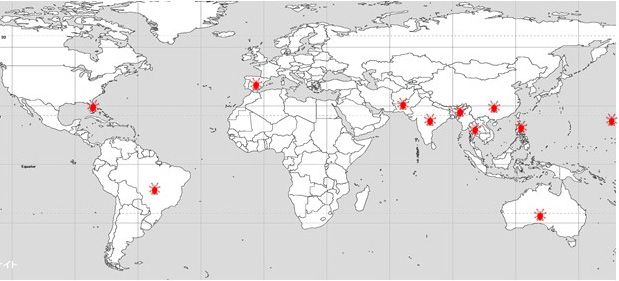
The lychee erinose mite (LEM), Aceria litchii (Keifer) (Acari: Eriophyidae), is one of the most important pests of lychee (Litchi chinensis Sonn., Sapindaceae). This eriophyid mite pest is native to Asiaand has been reported in India (Sharma 1985), Pakistan (Alam and Wadud 1963), Bangladesh (Haque et al. 1998), Thailand (Keifer and Knorr 1978), China and Taiwan (Huang 1967), Hawaii (Keifer 1943), and Australia (Pinese 1981) (Figure 1). More recently, LEM was found in Brazil (Raga et al. 2010; Fornazier et al. 2014), where it has spread to all major lychee-producing areas and has caused an estimated 70–80% yield reduction and a 20% increase in production costs (Navia et al. 2013). Prasad and Singh (1981) also reported an 80% yield reduction in India caused by LEM. In February 2018, LEM was found in Lee County, Florida, and since then it has spread to several counties in central and south Florida (Carrillo et al. 2020). As a result of this incursion, the Florida Department of Agriculture and Consumer Services, Division of Plant Industry (FDACS-DPI),established an eradication program and a quarantine in Lee county.
Credit: undefined
Synonymy
Eriophyes litchii Keifer (1943)
Identification, Damage Symptoms and Host Range
As a typical eriophyid mite, LEM is vermiform (tubular-shaped) and has two pairs of legs. It is approximately 150 µm in length and cannot be seen with the naked eye (Figure 2).

Credit: Dr. G. Bauchan†, SEL-USDA
LEM uses a series of stylets (Figure 3) to pierce and feed on leaf epidermal cells. Punctured cells often die but surrounding epidermal cells undergo morphological alterations (structural changes), resulting in the enlargement (hyperplasia) of leaf hairs (trichomes), referred to as “erinea” (Karioti et al. 2011). The enlargement and excessive branching of leaf hairs provide mites with a favorable habitat, protecting them from natural enemies and environmental adversities.
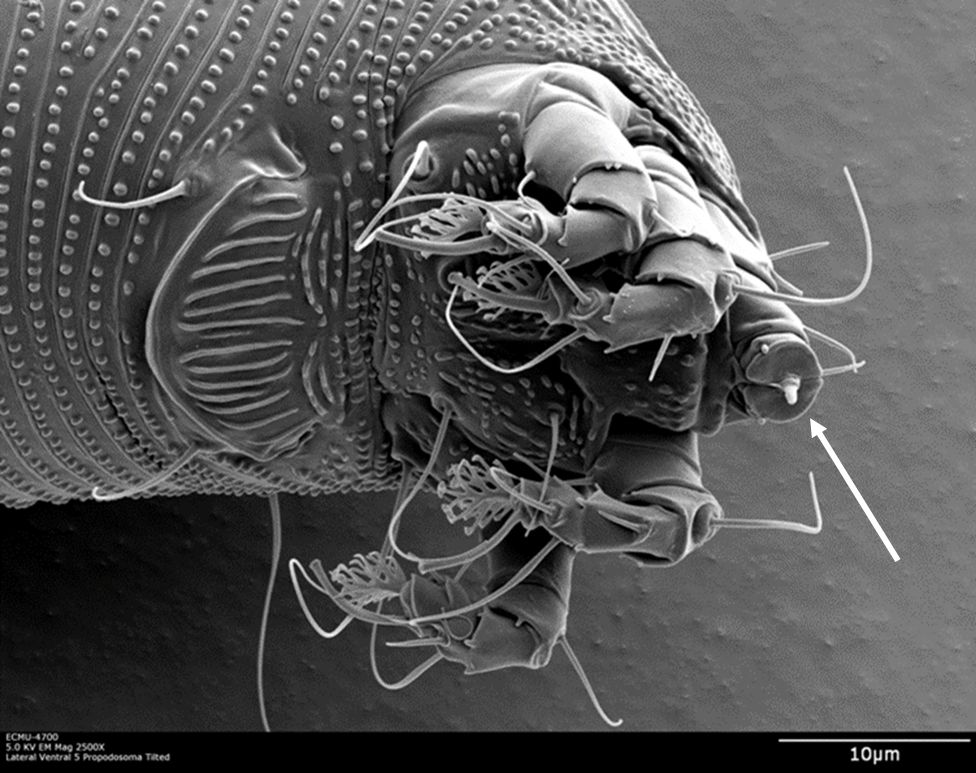
Credit: Dr. G. Bauchan†, SEL-USDA
Erinea develop initially on the lower side of the leaves, presenting a white/transparent coloration, and causing leaves to become distorted or curled (Figure 4a). However, at this stage of the infestation, a color change of the leaf can also be visible on the upper side (Figure 4b). As LEM populations grow, the erinea change color, thickness, and density. Dense white erinea (Figure 4c) have fewer mites present in comparison to amber colored erinea (Figure 4d). Erinea of dark brown or black color (Figure 4e) have little to no mites present. At this stage, the mites have overexploited the leaf and have dispersed in search of a new flush within the same plant. Erinea may also develop on petioles, stems, panicles, flower buds, and fruit. They may vary in size, shape, and color (Figure 5). Heavy infestations have typically multiple, much larger erinea that may vary in maturation due to the time it takes for the mites to disperse within the plant (Nishida and Holdaway 1955; Sabelis and Bruin 1996). The exact mechanism lying under the erinea formation remains unknown. Research currently conducted at UF/IFAS Tropical Research and Education Center (TREC) aims at understanding the mite-plant interactions and investigating how the erinea are formed.

Credit: Alexandra M. Revynthi, UF/IFAS TREC

Credit: Alexandra M. Revynthi and Daniel Carrillo, UF/IFAS TREC, Amy Roda, USDA-APHIS
LEM is highly host-specific and is well documented as a pest of lychee (Oldfield 1996). There is a single report of LEM attacking longan (Dimocarpus longan) in Taiwan (Huang 2008); however, this most likely is an anomaly. The author either confused LEM with a closely related mite, Aceria dimocarpi (Kuang), that attacks longan, or the mite was intercepted on longan but without causing any damage. In Brazil and Florida, longan planted alongside lychee infested with LEM never developed symptoms. Young lychee trees are more susceptible to LEM infestations, and some lychee varieties may be more susceptible than others (Arantes et al. 2017). However, all lychee varieties grown in Florida have shown susceptibility to LEM.
Life Cycle and Dispersal
LEM eggs are only laid after the erinea formation and are located at the base of erinea (Figure 6). They are approximately 32 μm in length and hatch into larvae within three to four days. Larvae are on average 49 μm in length, and they molt to nymphs after two to three days. LEM nymphs are on average 80 μm long and molt to adults within five to seven days (Alam and Wadud 1963). The development from egg to adult takes approximately 14 days depending on environmental conditions (Jeppson et al. 1975). Multiple, overlapping generations can occur over the course of one year. Population growth is favored by new growth on trees during moderately hot and dry periods. High temperature, high relative humidity, and heavy rainfall were unfavorable for LEM development in Pakistan (Alam and Wadud 1963), but in Brazil no correlation was found between LEM population densities and these environmental factors (Azevedo et al. 2014).
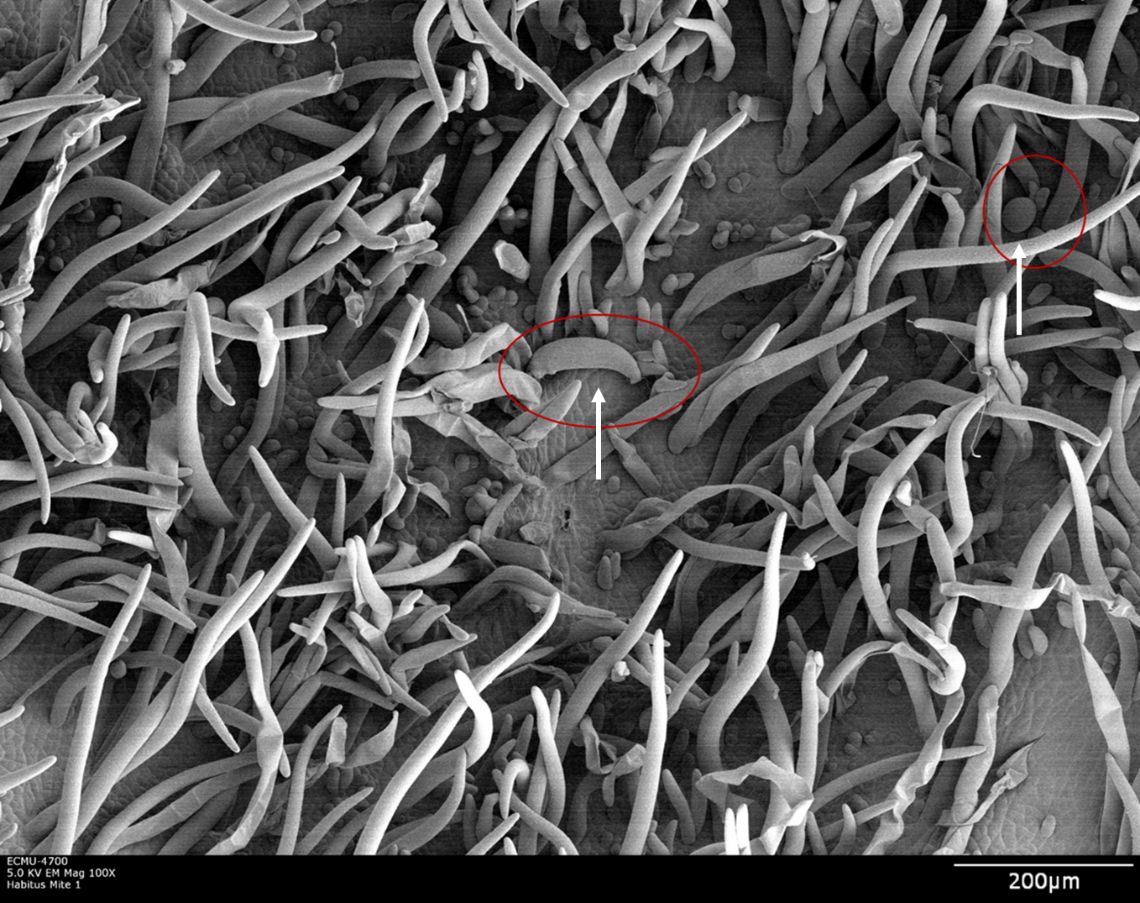
Credit: Dr. G. Bauchan, SEL-USDA
The mites prefer to feed on young new flush, which they infest by walking from leaf to leaf among the new flush (Alam and Wadud 1963; Azevedo et al. 2013) (Figure 4). For long-range dispersal, LEM disperses by phoresy, “hitching a ride” by attaching to the bodies of honeybees during the blooming season (Waite and McAlpine 1992; Waite 1999). Mites can also disperse using wind currents or by the plant propagation through air-layers. Air-layering is a method of propagating new trees from stems still attached to the parent tree. Air-layers produced from LEM-infested parent trees can facilitate movement of the mite to new locations where the air-lyaers are taken.
Management
Frequent and regular monitoring of trees should be conducted for early detection of LEM infestations. Any shoots with emerging stems and leaves and/or panicles are especially susceptible to LEM attack. Monitoring for the presence of LEM requires regular and careful inspections of the foliage to detect symptoms, especially around the time when trees are expected to flush or are actively flushing. The mites cannot be seen with a hand lens; high magnification (stereomicroscope) is required.
Chemical control in combination with pruning is the main approach that is followed worldwide for managing LEM infestations. Several active ingredients have shown some efficacy (Schulte et al. 2007; Azevedo et al. 2013), but satisfactory control can only be achieved through carefully timed treatments to protect the new flush (Waite 2005; Picoli et al. 2010). In other regions with LEM, foliar applications are made of various miticides (Castro et al. 2018; Nadhida and Holdway 1955; Schulte et al. 2007; Waite 2005). However, most of the products are not approved for use in the United States. All these miticides are prophylactic (applied before an LEM infestation) and do not kill LEM once the erineum is established.
Until recently, only two acaricides, bifenazate and abamectin, were registered for use in lychee in Florida. Bifenazate is an acaricide used against many species of spider mites, but it is not known to be active on gall/rust, broad, or flat mites (Cloyd 2004). Abamectin is used for a broader spectrum of mite species, including multiple gall/rust species such as the tomato russet mite (Aculops lycopersici) and the citrus bud mite (Aceria sheldoni). This acaricide, however, can only be applied twice per year on lychee and is not compatible with sulfur. In early 2021, EPA made available a Special Local Needs label (EPA Registration: 70506-187), which was approved by the FDACS-DPI, for the use of sulfur (Microthiol Disperss®, UPL, King of Prussia, PA, USA) for control of LEM on lychee.
The main cultural control strategy against LEM consists of removing and burning infested branches (Waite 2005; Castro et al. 2018). Pruning must be followed by sulfur applications to protect the new flush (see below). Pruning without supplementary sulfur applications may aggravate LEM's spread. Cultural practices are combined with repeated sulfur applications to prevent colonization of the new shoots and leaves by LEM. Once the trees are pruned, a sulfur application of Microthiol Disperss® is made. Sulfur is applied to run-off to all parts of the tree, including the trunk. Subsequent sulfur applications start with the emergence of the new flush and are repeated every 15 days until the leaves have hardened and the tree has stopped producing new flush. Phytotoxicity trials conducted at TREC showed little to no phytotoxicity caused by sulfur application. However, during periods of high temperatures sulfur may burn foliage and fruit. Avoid sulfur applications at temperatures over 90°F for three consecutive days. Additionally, sulfur products are not compatible with oil sprays.
Havesting lychee entails pruning off the fruit-ladened panicals. After harvest, pruning the trees is recommended. The purpose of the postharvest pruning is to control the tree size, maintain canopy light exposure and fruit production along the sides of the tree, synchronize the flush and development of stems, make cultural practices such as foliar pest control and nutrient applications more efficaceous, and reduce the potential for mechanical damage as a result of tropical storms and hurricanes. Note that postharvest pruning without supplementary sulfur applications may aggravate LEM's spread.
Due to current regulations, lychee producers at LEM-positive locations may ship lychee fruit to other non-lychee-producing states but are not allowed to sell the fruit in the state of Florida. In response to these limitations, Revynthi et al. (2020) developed a postharvest treatment using paraffinic oil dips that can be used to disinfest lychee fruit of LEM. This postharvest treatment did not result in fruit quality reduction. FDACS has approved this postharvest treatment, which allows growers in Lee County and other quarantine areas to move lychee fruit within the state of Florida.
Several natural enemies have been reported in association with LEM in India (Lall and Rahman, 1975; Thakur and Sharma 1990), Australia (Schicha 1987; Waite and Gerson 1994), Brazil (Picoli et al. 2010; Azevedo et al. 2014) and China (Waite and Hwang 2002). However, predation on LEM was only confirmed for a few species of predatory mites, including Amblyseius largoensis (Muma) (Acari: Phytoseiidae) in China (Cheng et al 2015), and Phytoseius intermedius Evans & MacFarlane (Acari: Phytoseiidae) in Brazil (Evans and Macfarlane 1961; Azevedo et al. 2014). Phytoseius intermedius was the predator most frequently found associated with LEM, and detailed studies determined that LEM is a suitable prey for this predator (Azevedo et al 2014). However, despite the frequent occurrence of P. intermedius, this predator was unable to prevent visible damage to the trees (Azevedo et al. 2014). Picoli and Vieira (2013), who reported the mite fungal pathogen Hirsutella thompsonii (Fischer) naturally infecting LEM in Brazil, suggested that the erinea may facilitate the development of the fungus and its persistence on the plants. Three phytoseiid predators have been found associated with LEM in Florida, Phytoseius woodburyi De Leon, A. largoensis and Euseius mesembrinus (Dean) (Acari: Phytoseiidae). However, their potential as biological control agents of LEM has not been assessed.
Please contact FDACS-DPI (tel: 1-888-397-1517) if you suspect a LEM infestation and contact your local UF/IFAS Extension agent for more information. Also visit the UF/IFAS Tropical Research and Education Center Lychee erinose mite website for current information (https://trec.ifas.ufl.edu/Lychee-Erinose-Mite/).
References
Alam, Z. M. and M. A. Wadud. 1963. "On the Biology of Litchi Mite, Aceria litchi Keifer (Eriophyidae, Acarina) in East Pakistan." Pakistan Journal of Medical Sciences 15:232–240
Arantes, R. F., D. J. de Andrade, and I. Amaral. MABG 2017. "Evaluation of Litchi Varieties Seeking Sources Resistant to Aceria litchi Mite." Revista Brasileira de Fruticultura 39:1–7. https://doi.org/10.1590/0100-29452017
Azevedo, L. H., G. J. Moraes, P. T. Yamamoto, and O. Z. Zanardi. 2013. "Development of a Methodology and Evaluation of Pesticides Against Aceria litchii and Its Predator Phytoseius intermedius (Acari: Eriphyidae, Phytoseiidae)." Journal of Economic Entomology 106:2183–2189. https://doi.org/10.1603/EC13026
Azevedo, L. H., E. Y. Maeda, M. M. Inomoto, and G. J. De Moraes. 2014. "A Method to Estimate the Population Level of Aceria litchii (Prostigmata: Eriophyidae) and a Study of the Population Dynamics of This Species and Its Predators on Litchi Trees in Southern Brazil." Journal of Economic Entomology 107:361–367. https://doi.org/10.1603/EC13337 Bolton, S. J., H. Klompen, G. R. Bauchan, and R. Ochoa. 2014. "A New Genus and Species of Nematalycidae (Acari: Endeostigmata)." Journal of Natural History 48:1359–1373. https://doi.org/10.1080/00222933.2013.859318
Carrillo, D., L. F. Cruz, A. M. Revynthi, R. E. Duncan, G. R. Bauchan, R. Ochoa, P. E. Kendra, and S. J. Bolton. 2020. "Detection of the Lychee Erinose Mite, Aceria litchii (Keifer) (Acari: Eriophyidae) in Florida, USA: A Comparison with Other Alien Populations." Insects 11:235. https://doi.org/10.3390/insects11040235
Castro, B. M. C., A. Plata-Rueda, W. Meloni Silva, C. W. Guimarães de Menezes, C. F. Wilcken, and J. C. Zanuncio. 2018. "Manejo del ácaro Aceria litchii (Acari: Eriophyidae) en Litchi chinensis." Revista Colombiana de Entomologia 44:2. https://doi.org/10.25100/socolen.v44i1.6528
Cheng, L., X. Zhang, L. Sha, A. Lu, and P. Chen. 2005. "Functional and Numerical Response of Amblyseius largoensis to Aceria litchii." Chinese Journal of Tropical Crops 8: 52–59
Cloyd, R. A. 2004. "All Miticides Are Not Created Equal." Home, Yard and Garden Pest Newsletter. Issue No. 17. University of Illinois Extension
Evans, G. O., and D. Macfarlane. 1961. "A New Mite of the Genus Phytoseius Ribaga (Acari : Mesostigmata)." Annals and Magazine of Natural Hististory 4:587–588. https://doi.org/10.1080/00222936108651183
Fornazier, M. J., D. D. S. Martins, D. L. Fornazier, L. H. Azevedo, J. S. Zanuncio Jr., and J. C. Zanuncio. 2014. "Range Expansion of the Litchi Erinose Mite Aceria Litchii (Acari: Eriophyidae) in Brazil." Florida Entomologist 97:846–848. https://doi.org/10.1653/024.097.0276
Haque, M. M., B. C. Das, M. Khalequzzaman, and S. Chakrabarti. 1998. "Eriophyoid Mites (Acari: Eriophyoidea) from Bangladesh." Oriental Insects 32:35–40. https://doi.org/10.1080/00305316.1998.10433765
Huang, K. 2008. "Aceria (Acarina: Eriophyoidea) in Taiwan: Five New Species and Plant Abnormalities Caused by Sixteen Species." Zootaxa 1829:1–30.
Huang, T. 1967. "A Study on Morphological Features of Erinose Mite of Litchi (Eriophyes litchii Keifer) and an Observation on the Conditions of its Damage." Plant Protection Bulletin 9:35–46.
Jeppson, L. R., H. H. Keifer, and E. W. Baker. 1975. Mites Injurious to Economic Plants. University of California Press, Berkley and Los Angeles, pp. 614.
Karioti, A., G. Tooulakou, A. R. Bilia, G. K. Psaras, G. Karabourniotis, and H. Skaltsa. 2011. "Erinea Formation on Quercus ilex Leaves: Anatomical, Physiological and Chemical Responses of Leaf Trichomes against Mite Attack." Phytochemistry 72:230–237. https://doi.org/10.1016/j.phytochem.2010.11.005
Keifer, H. H. 1943. "Eriophyid Studies XIII." State Calif Dep Agric Bull 32:212–222.
Keifer, H. H. and L. C. Knorr. 1978. "Eriophyid Mites of Thailand." Plant Protection Service Technical Bulletin 38, Bangkok, pp. 1–36
Lall, B. S., and M. F. Rahman. 1975. "Studies on the Bionomics and Control of the Erinose Mite Eriophyes litchii Keifer (Acarina: Eriophyidae)." Pesticides 9:49–54.
Navia, D., A. L. M. Júnior, M. G. C. Gondim Jr., R. S. de Mendonça, and P. R. V. da Silva Pereira. 2013. Recent Mite Invasions in South America." Potential Invasive Pests of Agricultural Crops 251–287
Nishida, T. and F. G. Holdaway. 1955. "The Erinose Mite of Lychee." Circular No. 48, Hawaii Agriculture Experiment Station, Honolulu, Hawaii, pp. 10.
Oldfield, G. N. 1996. "Diversity and Host Plant Specificity." In Eriophyoid Mites—Their Biology, Natural Enemies and Control. World Crop Pests vol 6, edited by E. E. Lindquist, M. W. Sabelis, and J. Bruin. Elsevier Science Publishing, Amsterdam, pp. 199–216.
Picoli, P. R. F., and M. R. Vieira. 2013. "Primeiro relato de atividade patogênica de Hirsutella thompsonii (Fischer) sobre o ácaro-da-erinose-da-lichia Aceria litchii (Keifer)." Semina: Ciências Agrárias 34:187–190. http://dx.doi.org/10.5433/1679-0359.2013v34n1p187
P. R. F. Picoli, M. R. Vieira, E. A. da Silva, M. S. de Oliveira da Mota. 2010. "Ácaros Predadores Associados Ao Ácaro-Da-Erinose Da Lichia." Pesquisa Agropecuária Brasileira 45:1246–1252. https://doi.org/10.1590/S0100-204X2010001100003
Pinese, B. 1981. "Erinose Mite—A Serious Litchi Pest." Queensland Agricultural Journal 107:79–81
Raga, A., J. L. de Carvalho Mineiro, M. E. Sato, G. J. Moraes, and C. H. W. Flechtmann. 2010. "Primeiro relato de Aceria litchii (Keifer) (prostigmata: eriophyidae) em plantas de lichia no Brasil." Revista Brasileira de Fruticultura 32:628–629. https://doi.org/10.1590/S0100-29452010005000046
Revynthi, A. M., R. E. Duncan, C. Mannion, P. E. Kendra, and D. Carrillo. 2020. "Post-Harvest Paraffinic Oil Dips to Disinfest Lychee Fruit of Lychee Erinose Mite." Florida Entomology 103:299–301. https://doi.org/10.1653/024.103.0224
Sabelis, M. W., and J. Bruin. 1996. "Evolutionary Ecology: Life History Patterns, Food Plant Choice and Dispersal." In Eriophyoid Mites—Their Biology, Natural Enemies and Control, edited by E. E. Lindquist, M. W. Sabelis, and J. Bruin. Elsevier Science Publishing, Amsterdam, The Netherlands, pp. 329–366.
Schicha, E. 1987. Phytoseiidae of Australia and Neighboring Areas. Indira Pub. House
Schulte, M. J., K. Martin, and J. Sauerborn. 2007. "Efficacy of Spiromesifen on Aceria litchii (Keifer) in Relation to Cephateuros virescens Kunze Colonization on Leaves of Litchi (Litchi chinensis Sonn.)." Journal of Plant Diseases and Protection 114:133–137
Thakur, A. P., and D. D. Sharma. 1990. "Influence of Weather Factors and Predators on the Populations of Aceria litchii Keifer." Indian Journal of Plant Protection 18:109–112
Waite, G. K. 1999. "New Evidence Further Incriminates Honey-Bees as Vectors of Lychee Erinose Mite Aceria litchii (Acari: Eriophyiidae)." Experimental and Applied Acarology 23:145–147. https://doi.org/10.1023/A:1006002611074
Waite, G. K. 2005. "Pests." In Litchi and Longan: Botany, Production and Uses edited by C. M. Menzel and G. K. Waite. pp. 237–259
Waite, G. K., and U. Gerson. 1994. "The Predator Guild Associated with Aceria litchii (Acari: Eriophyidae) in Australia and China." Entomophaga 39:275–280. https://doi.org/10.1007/BF02373032
Waite, G. K., and J. D. McAlpine. 1992. "Honey Bees as Carriers of Lychee Erinose Mite Eriophyes litchii (Acari: Eriophyiidae)." Experimental and Applied Acarology 15:299–302. https://doi.org/10.1007/BF01246570
Waite, G. K., and J. S. Hwang. 2002. "Pests of Litchi and Longan" In Tropical Fruit Pests and Pollinators: Biology Economic Importance, Natural Enemies and Control edited by J. E. Pena, J. L. Sharp, and M. Wysoki M. CAB International, Wallingford, UK, pp. 331–359




