Lilioceris egena (Weise) (Coleoptera: Chrysomelidae: Criocerinae)—Biological Control Agent of Air Potato Vine
This publication provides an in-depth profile of the air potato bulbil beetle (suggested common name), a new biological control agent of the air potato vine. It is intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The air potato vine (Dioscorea bulbifera L.) is a perennial, twining herbaceous vine capable of climbing tree canopies and displacing native vegetation (Morton 1976; Schultz 1993; Schmitz et al. 1997). This vine grows rapidly, attaining lengths of 20 m (65 ft) or greater, forming dense blankets over extensive areas such as tropical and subtropical hammocks, marshes, floodplain forests, waterways, and urban lots (Bell and Taylor 1982; Center et al. 2013; Rayamahji et al. 2016) (Figure 1). It was first introduced to Florida in the early 1900s and since then has spread through all 67 counties (Wheeler et al. 2007; Croxton et al. 2011; EDDMaps 2022). Currently, the vine is present in several southeastern states (Alabama, Georgia, Louisiana, Mississippi, South Carolina, Texas) and Hawaii (Figure 2). It is listed as a Category I invasive plant by the Florida Invasive Species Council (FISC) and is a regulated plant by the Florida Department of Agriculture and Consumer Services (FDACS).

Credit: Rosemary Murray, FDACS-DPI
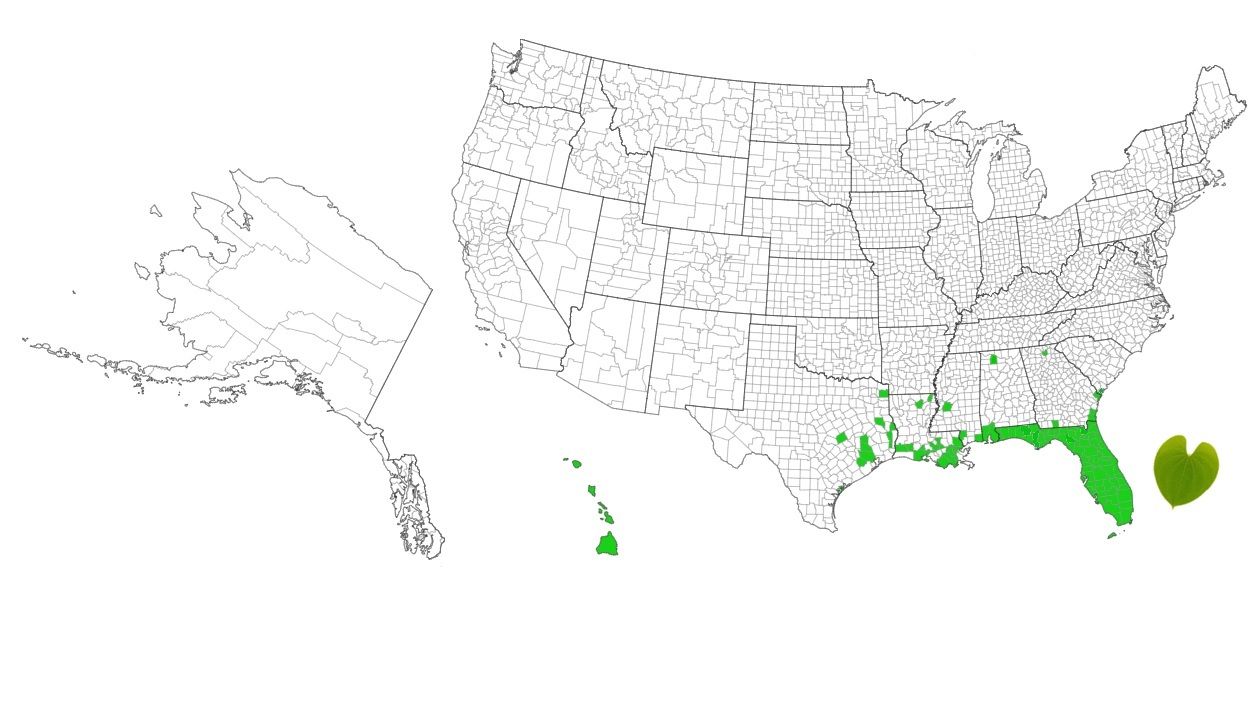
Credit: Center for Invasive Species and Ecosystem Health, EDDMaps, University of Georgia, GA
In 2012, a highly specialized leaf-feeding beetle, Lilioceris cheni Gressitt and Kimoto, was released as biological control agent of air potato (Pemberton and Witkus 2010; Diaz et al. 2013; Center et al. 2015; Enloe and Langeland 2021). Although Lilioceris cheni reduces the severity of air potato infestations in agricultural and natural ecosystems (Rayamajhi et al. 2019), the production of vegetative propagules (i.e., bulbils) continues, especially in South Florida (Overholt et al. 2016). Air potato expands its geographic range only through bulbils, as flowers are extremely rare and seed production has never been observed in Florida (Hammer 1998). In 2021, a bulbil-feeding beetle, Lilioceris egena, was approved by the USDA-APHIS-PPQ (United States Department of Agriculture, Animal & Plant Health Inspection Service, Plant Protection and Quarantine) to be released in Florida as an additional biological control agent of air potato. The damage caused by Lilioceris egena to air potato bulbils is expected to couple with defoliation by Lilioceris cheni to further reduce the number of air potato vines in Florida. This document provides information on the distribution, description, biology and ecology, host specificity, and importance of Lilioceris. egena.
Synonyms
- Crioceris egena Weise, 1922
- Crioceris coomani Pic, 1928
Distribution
Lilioceris egena is native to Southeast Asia. Country records include China (Anhui, Fujian, Hainan, Hong Kong, Sichuan, and Yunnan Provinces), India (Assam, Karnataka, and Uttarhand), Laos (Vientianne Province), Nepal (Province 3), Vietnam (Tây Nihn and Ho Chi Minh Provinces), and Singapore (Tishechkin et al. 2011; Warchalowski 2011).
Description
Egg
The egg of Lilioceris egena is initially whiteish, with an oval shape, and approximately 1 mm long. As the embryo develops it becomes creamy yellow (Figure 3). Upon hatching, the eggs turn greenish-gray.

Credit: Jeff Lotz and Rosemary Murray, FDACS-DPI
Larva
The larva of Lilioceris egena goes through four instars until it reaches the pupal stage. Newly hatched larvae are cloudy translucent with black legs, head capsule, and thoracic plates. Later instars appear grayish to reddish in color (Figure 4).

Credit: Jeff Lotz and Rosemary Murray, FDACS-DPI
Pupa
The pupa of Lilioceris egena is peach-colored and motionless. Fully developed larvae exit the bulbil and crawl to the soil where they produce a white oral substance that adheres to soil particles. The oral secretion hardens into a foam-like cocoon called puparium (plural = puparia). Puparia can be observed approximately 10 days after eggs hatching. Occasionally some larvae pupate without forming a puparium, but typically, pupae are found concealed within the puparium (Figure 5).
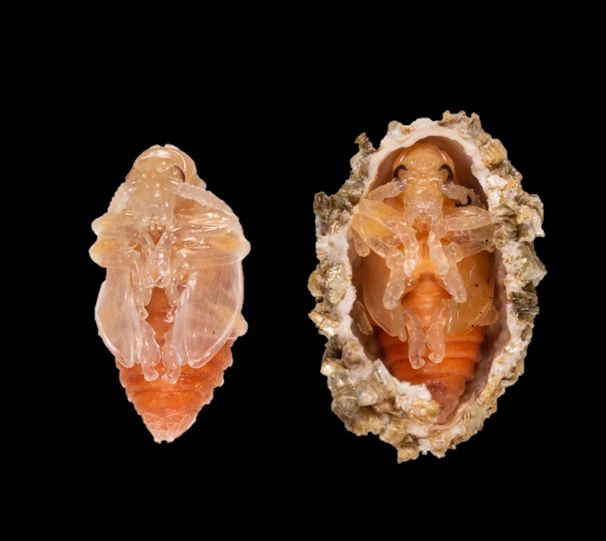
Credit: Jeff Lotz and Rosemary Murray, FDACS-DPI
Adult
The adult Lilioceris egena is approximately 10 mm long and 5 mm wide. The head, thorax, abdomen, and legs are black, whereas the wing covers (i.e., elytra) are brownish - orange to bright red (Figure 6). Similar to other Criocerinae species, Lilioceris egena is elongate, with a rectangular-shaped abdomen, slim thorax, and a narrow head with bulging eyes. Currently in the United States, there are only two Lilioceris species (none of them native): the invasive Lilioceris lilii (Scopoli), common pest of lilies, and the air potato leaf beetle, Lilioceris cheni. The key character to distinguish Lilioceris lilii from Lilioceris cheni and Lilioceris egena is its bright red thorax. However, it is virtually impossible to differentiate Lilioceris cheni from Lilioceris egena in the field. These species were distinguished by Center et al. (2013) based on behavioral (bulbil feeding and oviposition) and morphological characters, mainly the structure of male genitalia.

Credit: Jeff Lotz and Rosemary Murray, FDACS-DPI
Biology and Ecology
Adults bore into and feed mainly on bulbils (Figure 7a) although occasionally can feed on air potato leaves. Females lay eggs on the surface of- and within- fallen bulbils. Sometimes, although extremely rare, females may oviposit on air potato leaves. Eggs, with a developmental time of approximately 5.5 days, are laid either individually or in clusters of 2 to 15 eggs. A single female can produce an average of 8 eggs/day and more than 900 eggs during her lifespan. Newly hatched larvae are unable to enter the bulbil on their own. Instead, they rely on tunnels excavated by adults during feeding. The larval stage lasts around 16 days. Mature larvae (3rd and 4th instars) can exit and re-enter the bulbils by digging additional holes in their surface (Figure 7b). Several larvae can be found feeding in a single bulbil causing damage so severe that its interior (i.e., primary meristematic region) appears liquified; inhibiting its ability to sprout (Figure 7c). It is possible that bulbil collapse is due to bacterial and/or enzymatic activity that alter the quality and functional properties of the bulbil. When a bulbil is no longer viable, laboratory observations have shown that larvae can migrate to nearby bulbils. However, in field conditions, factors like flooding and predators such as birds, lizards, and ants may play a major role in larval survival. The puparium, which is the protective casing for the pupal stage, can be found loosely in the soil or in clusters of 2 to 5 individuals commonly attached to the underside of the bulbils. The pupal stage lasts approximately 12 days. The total developmental time from egg to adult is approximately 35 days. Adults typically live for 120 days, although some may survive up to 200 days. Recently emerged adults can survive up to three weeks with water only.

Credit: Jeff Lotz and Rosemary Murray, FDACS-DPI
Similar to Lilioceris cheni, cold temperatures appear to reduce the oviposition of Lilioceris egena. In laboratory experiments at the Florida Department of Agriculture and Consumer Services (FDACS), beetles held at 16°C (~60°F) showed a significant reduction in egg numbers compared to those kept at 24°C (~75°F). In Florida, low temperatures occur between December and March (FAWN 2022). It is unknown how Lilioceris egena will perform during the winter months, but a reduction in Lilioceris egena activity is expected. More research is needed to determine how low temperatures, in natural settings, would influence the activity and oviposition behavior of ilioceris. egena.
Host Specificity
Several studies conducted at the USDA ARS - Invasive Plant Research Laboratory determined the specificity of Lilioceris egena against air potato. Host range testing included 82 plant species belonging to 46 families and 25 orders. Plants within the Dioscoreaceae (15) were chosen based on economic importance and major taxonomic sections of the family present in Florida and the West Indies. Additionally, plant species known to exhibit storage organs (i.e., roots/corms/expanded rhizomes/tubers) of economic importance were also included.
The longevity and survival of Lilioceris egena were significantly greater on air potato compared to the longevity and survival on non-target plants and non-target storage organs. Lilioceris egena laid eggs only on air potato. Interestingly, females would hold their eggs while on air potato leaves only to lay eggs soon after they were transferred to bulbils. These results indicated a high degree of specificity towards bulbils. Further experiments using larvae revealed that their developmental success was greater on bulbils than on air potato leaves. Newly hatched larvae forced to feed and develop on any plant species aside from air potato exhibited high mortality. Adult feeding on non-target plants was minimal compared to the target plant (air potato). Altogether, the results indicated that Lilioceris egena is a highly specialized biological control agent of air potato (Stewart 2021).
Importance as Biological Control Agent
It is expected that larvae and adults of Lilioceris egena will have a direct effect on air potato abundance. The strong preference of Lilioceris egena for bulbils should lead to large numbers of damaged bulbils, reducing their quality, and hence their ability to sprout. Areas that once were covered by air potato will recover their original biodiversity and ecological functions. Finally, the need for other control techniques such as cultural and chemical should be reduced. Long-term surveys are currently ongoing in North, Central, and South Florida to determine the establishment of Lilioceris egena and its impact on air potato. Further reducing the dominance of air potato in natural habitats will diminish its chances to spread in Florida and the southern United States. More information on biological control of air potato can be found in Diaz et al. (2013) and Center et al. (2015). Additionally, information about air potato vine management can be found in Kraus and Murray (2021).
The authors would like to acknowledge two anonymous reviewers who provided feedback on an early version of the article.
References
Bell, C. R., and B. J. Taylor. 1982. “Florida wildflowers and roadside plants.” Laurel Hill Press, Chapel Hill, North Carolina.
Center, T. D., M. Rayamajhi, F. A. Dray, P. M. Madeira, G. Witkus, E. Rohrig, E. Mattison, E. Lake, M. Smith, J. Zhang, M. Purcell, A. Konstantinov, and D. Schmitz. 2013. “Host range validation, molecular identification and release and establishment of a Chinese biotype of the Asian leaf beetle Lilioceris cheni (Coleoptera: Chrysomelidae: Criocerinae) for control of Dioscorea bulbifera L. in the southern United States.” Biocontrol Science and Technology. 23:735–755. https://doi.org/10.1080/09583157.2013.790931
Center, T. D., W. A. Overholt, E. Rohrig, and M. Rayamajhi. 2015. “Classical biological control of air potato in Florida.” University of Florida - Institute of Food and Agricultural Sciences. EDIS. ENY-864.
Croxton, M., M. Andreu, D. Williams, W. Overholt, and J. Smith. 2011. “Geographic origins and genetic diversity of air-potato (Dioscorea bulbifera) in Florida.” Invasive Plant Science and Management. 4:22–30. https://doi.org/10.1614/IPSM-D-10-00033.1
Diaz, R., W. A. Overholt, and K. Hibbard. 2013. “Air potato leaf beetle (suggested common name), Lilioceris cheni Gressitt and Kimoto (Insecta: Coleoptera: Chrysomelidae: Criocerinae).” University of Florida - Institute of Food and Agricultural Sciences. EDIS. EENY-547. https://doi.org/10.32473/edis-in972-2013
EDDMaps. 2022. Early detection & distribution mapping system. The University of Georgia – Center for Invasive Species and Ecosystem Health. Available online at EDDMapS.
Enloe, F., and K. Langeland. 2021. “Invasive plants in natural areas: air potato (Dioscorea bulbifera).” University of Florida - Institute of Food and Agricultural Sciences. EDIS. SS AGR 164. https://doi.org/10.32473/edis-ag112-2021
FAWN. 2022. Florida Automated Weather Network. Available online at FAWN - Florida Automated Weather Network (ufl.edu).
Hammer, R. L. 1998. “Diagnosis: Dioscorea.” Wildland Weeds. 1:8–10.
Kraus, E. C., and R. Murray. 2021. “Air potato vine management guide: Combining management strategies to achieve eradication of the noxious weed Dioscorea bulbifera (air potato vine).” Circular. Florida Department of Agriculture and Consumer Services. FDACS-P-02163.
Morton, J. F. 1976. “Pestiferous spread of many ornamental and fruit species in south Florida.” Proceedings Florida State Horticultural Society. 89:348–353.
Overholt, W. A., M. Rayamajhi, E. Rohrig, S. Hight, F. A. Dray, E. Lake, M. Smith, K. Hibbard, G. P. Bhattarai, K. Bowers, R. Poffenberger, M. Clark, B. Curry, B. Stange, E. Calise, T. Wasylik, C. Martinez, and J. Leidi. 2016. “Release and distribution of Lilioceris cheni (Coleoptera: Chrysomelidae), a biological control agent of air potato (Dioscorea bulbifera: Dioscoreaceae), in Florida.” Biocontrol Science and Technology. 26:1087–1099. https://doi.org/10.1080/09583157.2016.1185090
Pemberton, R. W., and G. L. Witkus. 2010. “Laboratory host range testing of Lilioceris sp. near impressa (Coleoptera: Chrysomelidae) – a potential biological control agent of air potato, Dioscorea bulbifera (Dioscoreaceae).” Biocontrol Science and Technology. 20:567–587. https://doi.org/10.1080/09583150903531332
Rayamajhi, M. B., P. D. Pratt, P. W. Tipping, E. Lake, M. Smith, E. Rohrig, F. A. Dray, and T. D. Center. 2016. “Seasonal growth, biomass allocation, and invasive attributes manifested by Dioscorea bulbifera L. (Air-potato) plants generated from bulbils in Florida.” Invasive Plant Science and Management. 9:195–204. https://doi.org/10.1614/IPSM-D-16-00022.1
Rayamajhi, M. B., E. Rohrig, J. Leidi, C. Kerr, E. Salcedo, R. Poffenberger, M. Smith, E. Lake, F. A. Dray Jr, P. Pratt, P. Tipping, and T. Center. 2019. Herbivory by the biocontrol agent Lilioceris cheni suppresses propagule production and smothering ability of the invasive vine Dioscorea bulbifera. Biological Control. 130:1–8. https://doi.org/10.1016/j.biocontrol.2018.12.001
Schultz, G. E. 1993. “Element stewardship abstract for Dioscorea bulbifera air potato.” The Nature Conservancy, Davis, California.
Schmitz, D. C., D. Simberloff, R. L. Hofstetter, W. T. Haller, and D. Sutton. 1997. “The ecological impact of nonindigenous plants.” Pp. 39–61 in D. Simberloff, D. C. Schmitz, and T. C. Brown, eds., Strangers in Paradise: Impact and Management of Nonindigenous Species in Florida. Island Press, Washington, D.C.
Stewart, C. D. 2021. “Field release of the beetle Lilioceris egena (Coleoptera: Chrysomelidae) for classical biological control of air potato, Dioscorea bulbifera (Dioscoreaceae), in the continental United States.” Environmental Assessment. 79 pp.
Tishechkin, A. K., A. S. Konstantinov, S. Bista, R. W. Pemberton, and T. D. Center. 2011. “Review of the continental Oriental species of Lilioceris Reitter (Coleoptera, Chrysomelidae, Criocerinae) closely related to Lilioceris impressa (F).” ZooKeys. 103:63–83. https://doi.org/10.3897/zookeys.103.983
Warchalowski, A. 2011. “An attempt on a review of Lilioceris Reitter, 1913 – species from continental part of south-eastern Asia (Coleoptera: Chrysomelidae: Criocerinae).” Genus. 22:95–122.
Wheeler, G. S., R. W. Pemberton, and L. Raz. 2007. “A biological control feasibility study of the invasive weed-air potato, Dioscorea bulbifera L. (Dioscoreaceae): an effort to increase biological control transparency and safety.” Natural Areas Journal. 27:269–279. https://doi.org/10.3375/0885-8608(2007)27[269:ABCFSO]2.0.CO;2





