Introduction
Silicon (Si) is not classified as an essential plant nutrient, but it is considered a beneficial nutrient for sugarcane (Saccharum spp.) and rice (Oryza sativa L.) (Ma et al. 2002; Savant et al. 1999). Yields of both crops are increased when calcium (Ca) silicate slag is applied to soils low in soluble Si (Anderson et al. 1991; Elawad et al. 1982a, b; Fox et al. 1967; Snyder et al. 1986). Raid et al. (1992) measured average increases of 20% in sugarcane yield for two crop years and five cultivars following Ca silicate application at 3 tons/acre. Ca silicate application can benefit both crops in a rice-sugarcane rotation when it is applied prior to planting rice (Anderson et al. 1987). Mechanisms responsible for increased yield may include resistance to lodging through increased mechanical strength of cell walls, resistance to disease and insect damage, reduced water loss through evapotranspiration, improved phosphorus (P) metabolism, and reduced accumulation of toxic concentrations of heavy metals (Datnoff et al. 1997; Savant et al. 1999; Snyder et al. 1986).
Sugarcane responses to silicon fertilization have been reported in many areas of the world, particularly on weathered tropical and subtropical soils such as Oxisols, Ultisols, Entisols, and Histosols (Korndorfer and Lepsch 2001). Sugarcane is grown on approximately 400,000 acres in the Everglades Agricultural Area (EAA) of Florida, and 71% of this acreage is on organic soils (VanWeelden et al. 2021). Organic soils (Histosols) in the EAA near Lake Okeechobee tend to have relatively higher mineral content and soluble Si because of historic lake overflows. This includes the Torry muck soil series, which has > 35% mineral content that is predominately clay (McCollum et al. 1978). These organic soils with higher clay content should not require Si fertilization. Histosols located further from Lake Okeechobee have considerably lower mineral content and may contain very low levels of total and soluble Si. Sugarcane growing on these low mineral content soils can have strong yield responses to calcium silicate application (Gascho and Andreis 1974).
Since Ca silicate application requires a substantial grower investment, it is very important to consider the costs and benefits (Alvarez and Datnoff 2001). Leaf analysis can be a useful indicator of Si status, and optimum growth requires a suggested minimum leaf tissue concentration of 0.6% Si (McCray and Mylavarapu 2020). A recent survey of Florida sugarcane fields determined that an estimated 25% of surveyed fields on organic soils had production losses of > 10% due to insufficient leaf Si (McCray et al. 2010).
Recommendations were previously developed for Ca silicate application for rice production on Florida Histosols based on a soil test using 0.5 N acetic acid-extractable Si (Korndorfer et al. 2001), but prior to 2011 there was no soil-test Si calibration for sugarcane on these soils. This publication describes Ca silicate recommendations for sugarcane on organic soils developed using field studies at multiple locations and based on 0.5 N acetic acid-extractable Si.
Sugarcane Yield Response to Ca Silicate
Evaluations of sugarcane tonnage and sugar yield response to Ca silicate application were conducted at three small-plot locations and twelve paired commercial field locations on organic soils in the EAA (McCray and Ji 2011). In all experiments, the Si source was an electric furnace Ca silicate slag produced as a by-product of elemental P production. This slag contained approximately 20% Si on a dry weight basis (Table 1). Similar to previous studies with sugarcane in Florida (Anderson et al. 1991; Gascho and Andreis 1974), there were strong responses in tons cane/acre (TCA) and tons sugar/acre (TSA) to Ca silicate application. There was not a similar response to dolomite at the two test locations where dolomite was also tested, indicating that the TCA and TSA responses are attributable to applied Si and not pH increase or applied Ca. Also, leaf P concentration was not increased by Ca silicate application, so there was no indication of increased availability of P with slag application. Our findings agree with those of Elawad et al. (1982b) who determined that leaf P was directly related to the amount of P contained in the slag material. Phosphorus content in the Ca silicate applied in our study was relatively low (0.5% P; Table 1). There was no evidence supporting previous suggestions (Matichenkov and Calvert 2002; Matichenkov et al. 2002) that Ca silicate increases plant-available P.
Sugarcane yield responses to Ca silicate application ranged from 0 to 9 TCA/yr with relative yield reduced as much as 23% without application (McCray and Ji 2011). In addition to variable yield response explained by available soil Si, some variation in response to Si application can depend on site-specific factors such as disease or insect pressure that increased plant Si may help alleviate (Kvedaras et al. 2007; Raid et al. 1992). This study confirms the well-established role of Si as a beneficial nutrient in sugarcane (Savant et al. 1999) and emphasizes the need to maintain adequate soil Si availability for optimum growth (McCray et al. 2010).
Influence of Leaf Si and Extractable Soil Si on Sugar Yield
Relative sugar yield was used in the study to allow comparisons across crop sites and crop years with variation in soils, rainfall, and other growing conditions. Relative yields are determined by dividing the yield of a specific treatment by the highest yielding treatment in the experiment for each crop year and each location. Leaf Si concentration correlated strongly to relative sugar/acre (Figure 1). Sugar yield (the TSA parameter) was optimal with a leaf concentration of > 0.60% Si, and 0.95 and 0.80 relative yield levels corresponded to leaf concentrations of approximately 0.50% and 0.25%, respectively. This indicates the leaf Si critical level (0.95 relative yield) is 0.50%, which is similar to the value of 0.53% at which 0.95 relative sugarcane yield was determined in Australia (Berthelsen et al. 2003). These leaf Si values are in close agreement with those previously suggested using survey data from Florida (McCray et al. 2010), but are substantially lower than the suggested critical value of 1.00% leaf Si suggested by Anderson and Bowen (1990).
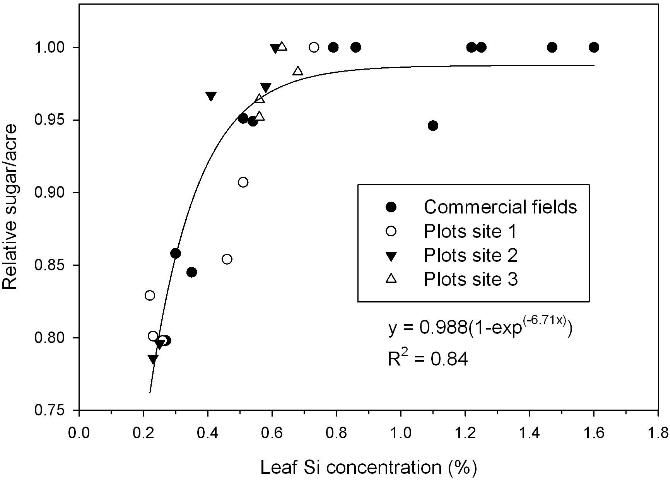
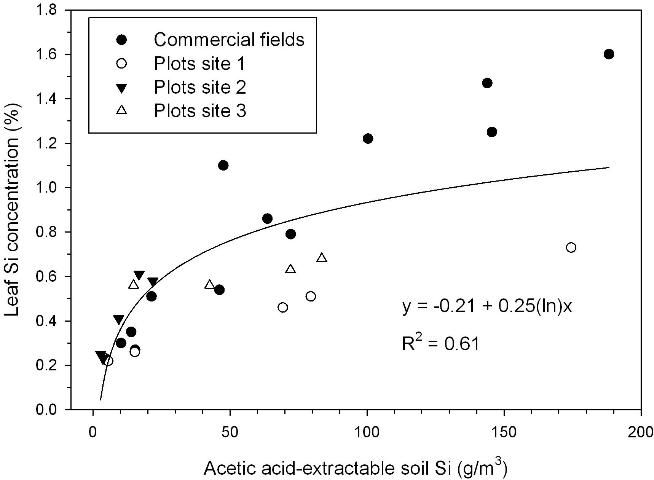
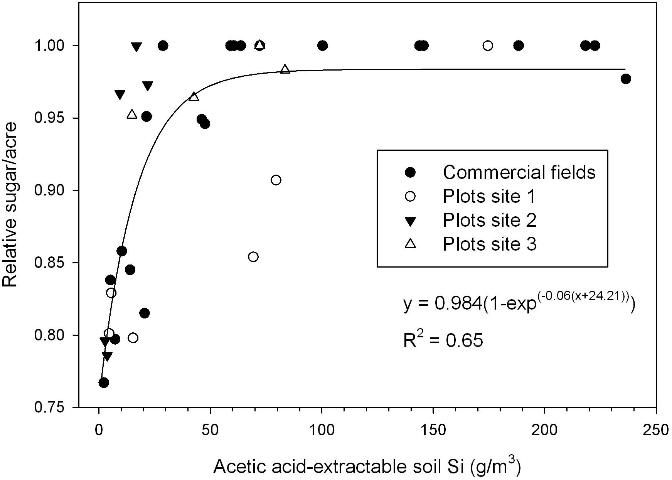
Acetic acid-extractable soil Si of approximately 26 g/m3 corresponded to a leaf Si concentration of 0.60% (Figure 2), the lower value required for optimum TSA (Figure 1). Relative sugar/acre related strongly to acetic acid-extractable soil Si, with relative yield of 0.95 reached in a regression model at 32 g Si/m3 for samples taken after the plant cane crop and including soils with and without Ca silicate amendment (Figure 3). The largest responses to Ca silicate were determined with acetic acid-extractable soil Si < 21 g/m3 prior to application. Also, at one of the small-plot test locations there was no significant TSA response to a first-time application of Ca silicate with acetic-acid extractable Si of 17 g/m3 (small-plot site 3). Leaf Si values and trends in TCA and TSA at this location indicated that soil Si availability was minimally adequate, so that a consistent response should be expected to first-time Ca silicate applications with acetic acid-extractable soil Si < 15 g/m3.
Ca Silicate Calibration Using the 0.5 N Acetic Acid Soil Test
Alvarez and Datnoff (2001) determined that application of Ca silicate to responsive soils could improve economic returns for Florida sugarcane growers. Predicting potential response in relation to soil-test Si levels is critical to making cost/benefit decisions, given the expense of Ca silicate application. Table 2 shows recommendations for Ca silicate application for sugarcane production on organic soils. In the proposed calibration, 2–3 tons Ca silicate/acre are recommended for soil-test Si values of < 15 g/m3. The high rate of 3 tons/acre has been effective in providing adequate Si in soils with low Si availability (McCray and Ji 2011; Raid et al. 1992). Lower Ca silicate rates (1.0–1.5 tons/acre) are recommended as maintenance applications for soils with acetic acid-extractable Si in the range of 16–25 g/m3. There could be a possible economic response to a first-time Si application within the soil-test Si range of 16–25, but leaf Si concentration should be used in addition to soil-test Si to determine the potential response. Soils that have previously responded to Si application that have soil-test Si > 15 are expected to decrease in available Si over time, hence the Ca silicate slag maintenance recommendation of 1.0–1.5 tons/acre for the acetic acid soil-test range of 16–25 g Si/m3. Recommended Ca silicate rates are expected to provide sufficient Si for at least a 3-year crop cycle. Also, these recommendations are similar to slag recommendations for rice (Korndorfer et al. 2001) and should provide adequate Si for rice in addition to the following sugarcane crops in rotation.
References
Alvarez, J., and L. E. Datnoff. 2001. "The Economics of Silicon for Integrated Management and Sustainable Production of Rice and Sugarcane. In Silicon in Agriculture, edited by L. E. Datnoff, G. H. Snyder, and G. H. Korndorfer, 221–39. Amsterdam: Elsevier.
Anderson, D.L., and J.E. Bowen. 1990. Sugarcane Nutrition. Atlanta, GA: Potash & Phosphate Institute.
Anderson, D.L., D.B. Jones, and G.H. Snyder. 1987. "Response of a Rice-sugarcane Rotation to Calcium Silicate Slag on Everglades Histosols." Agron. J. 79: 531–5.
Anderson, D.L., G.H. Snyder, and F.G. Martin. 1991. "Multi-year Response of Sugarcane to Calcium Silicate Slag on Everglades Histosols." Agron. J. 83: 870–4.
Berthelsen, S., A.D. Noble, G. Kingston, A. Hurney, A. Rudd, and A. Garside. 2003. Improving Yield and Ccs in Sugarcane through the Application of Silicon Based Amendments. Final Report – SRDC Project CLW009. Sugar Research and Development Corporation. CSIRO Land and Water PMB Aitkenvale, Townsville, Queensland.
Datnoff, L.E., C.W. Deren, and G.H. Snyder. 1997. "Silicon Fertilization for Disease Management of Rice in Florida." Crop Protection 16: 525–31.
Elawad, S.H., G.J. Gascho, and J.J. Street. 1982. "Response of Sugarcane to Silicate Source and Rate. I. Growth and Yield." Agron. J. 74: 481–4.
Elawad, S.H., J.J. Street, and G.J. Gascho. 1982. "Response of Sugarcane to Silicate Source and Rate. II. Leaf Freckling and Nutrient Content." Agron. J. 74: 484–7.
Fox, R.L., J.A. Silva, O.R. Younge, D.L. Plucknett, and G.D. Sherman. 1967. "Soil and Plant Silicon and Silicate Response by Sugarcane." Soil Sci. Soc. Am. Proc. 31: 775–9.
Gascho, G.J., and H.J. Andreis. 1974. "Sugarcane Response to Calcium Silicate Slag Applied to Organic and Sand Soils." International Soc. Sugarcane Technologists Proc. 15: 543–51.
Korndorfer, G.H., and I. Lepsch. 2001. "Effect of Silicon on Plant Growth and Crop Yield. In Silicon in Agriculture, edited by L. E. Datnoff, G. H. Snyder, and G. H. Korndorfer, 133–47. Amsterdam: Elsevier.
Korndorfer, G.H., G.H. Snyder, M. Ulloa, G. Powell, and L.E. Datnoff. 2001. "Calibration of Soil and Plant Silicon Analysis for Rice Production." J. Plant Nutrition 24: 1071–84.
Kvedaras, O.L., M.G. Keeping, F.R. Goebel, and M.J. Byrne. 2007. "Water Stress Augments Silicon-mediated Resistance of Susceptible Sugarcane Cultivars to the Stalk Borer Eldana Saccharina (Lepidoptera: Pyralidae)." Bulletin of Entomological Research 97: 175–83.
Ma, J.F., and E. Takahashi. Soil, Fertilizer, and Plant Silicon Research in Japan. Amsterdam: Elsevier 2002.
Matichenkov, V.V., B. Ande, P. Ande, D.V. Calvert, and E.A. Bocharnikova. 2002. "Effect of Silicon-rich Slag and Lime on Phosphorus Leaching in Sandy Soils." J. Am. Soc. Sugarcane Technologists 22: 9–20.
Matichenkov, V.V., and D.V. Calvert. 2002. "Silicon as a Beneficial Element for Sugarcane." J. Am. Soc. Sugarcane Technologists 22: 21–9.
McCollum, S.H., O.E. Cruz, L.T. Stem, W.H. Wittstruck, R.D. Ford, and F.C. Watts. 1978. Soil Survey of Palm Beach County Area, Florida. Washington, DC: Natural Resources Conservation Service, United States Department of Agriculture.
McCray, J.M., and S. Ji. 2012. "Calibration of Sugarcane Response to Calcium Silicate on Florida Histosols." J. Plant Nutrition 35: 1192–1209.
McCray, J.M., S. Ji, G. Powell, G. Montes, R. Perdomo, and Y. Luo. 2010. "Boundary Lines Used to Determine Sugarcane Production Limits at Leaf Nutrient Concentrations Less Than Optimum." Comm. Soil Sci. Plant Anal. 41: 606–22.
McCray, J.M., and R. Mylavarapu. 2020. Sugarcane Nutrient Management Using Leaf Analysis. Gainesville: University of Florida Institute of Food and Agricultural Sciences. Accessed October 26, 2015. https://edis.ifas.ufl.edu/ag345
Raid, R.N., D.L. Anderson, and M.F. Ulloa. 1992. "Influence of Cultivar and Amendment of Soil with Calcium Silicate Slag on Foliar Disease Development and Yield of Sugar-cane." Crop Protection 11: 84–8.
Savant, N.K., G.H. Korndorfer, L.E. Datnoff, and G.H. Snyder. 1999. "Silicon Nutrition and Sugarcane Production: A Review." J. Plant Nutrition 22: 1853–903.
Snyder, G.H., D.B. Jones, and G.J. Gascho. 1986. "Silicon Fertilization of Rice on Everglades Histosols." Soil Sci. Soc. Am. J. 50: 1259–63.
VanWeelden, M., S. Swanson, W. Davidson, M. Baltazar, and R. Rice. 2021. Sugarcane Variety Census: Florida 2020. Sugar J. 84(2):6-15.