Soil Acidity
Soil acidity refers to the concentration of active hydrogen ions (H+) in the soil. It is measured by an index called pH. Lower pH values are associated with more active hydrogen ions and more acidic soil conditions. The normal range of soil pH relative to its acidity or alkalinity is shown in Figure 1. A pH of 7 (as is the case for distilled water) is neutral because its acidity is equal to its alkalinity (H+ = OH-). There are a few native Florida soils with pH greater than 7. In general, most native Florida flatwoods soils are acidic, with pH around 4.5.
Effect of Nitrogen Fertilizer on Soil Acidity
Soil pH tends to decrease with repeated use of nitrogen (N) fertilizer because the N transformations that occur in the soil after fertilizer application generate acidity (H+). Soil microorganisms mediate the biological oxidation of ammonium (NH4+) to nitrate (NO3-). This transformation is called nitrification. Nitrification is a two-step process that generates hydrogen ions:
1. 2 NH4+ + 3 O2 ? 2 NO2- + 2 H2O + 4 H+ (This reaction is mediated by nitrosomonas bacteria.)
2. 2 NO2- + O2 ? 2 NO3- (This reaction is mediated by nitrobacter.)
When ammonium sulfate fertilizer is applied to soils, additional acidity may occur as a result of the fertilizer hydrolysis:
(NH4)2SO4 + 2H2O ? H2SO4 + 2 NH4OH
Increase in soil acidity following repeated application of N fertilizers is naturally countered to some extent by the soil buffering capacity (clay minerals and organic matter). The sandy flatwoods soils in South Florida tend to have low clay and organic matter content (< 2%) and generally low buffering capacities. Hence, soil acidity must eventually be neutralized by applying liming materials, which have the ability to increase soil pH. For example, 60 pounds of lime would be required to neutralize the acidity from 100 lb of ammonium nitrate. Similarly, 110 lb of lime would be required to neutralize the acidity from 100 lb of ammonium sulfate. The lime requirements for pasture should always be determined by a soil test performed by a reliable soil lab as well as by the forage species.
Effect of Soil pH on Nutrient Availability
In acid soils with pH less than 5, the availability of boron (B), molybdenum (Mo), and sulfur (S) is reduced, and nutrient uptake and forage production can be reduced. At soil pH < 4.0, there is also the potential for active aluminum (Al3+) to become toxic to plant roots. Additionally, under low pH (< 5) forages are susceptible to yellowing and damage by soilborne insects. Increasing soil alkalinity to pH greater than 7 (such as from repeated use of lime-stabilized biosolids) can also be harmful to forage grasses. High soil pH reduces the availability of micronutrients such as iron, manganese, zinc, copper, and cobalt, and creates forage production problems similar to increased acidity.
Mole Crickets and Changing Soil pH
Mole crickets may be a problem in established bahiagrass pastures in Florida. It usually begins with yellowing of the pasture in small or big patches. Later, affected pasture areas turn brown and die, which is normally associated with the burrowing and tunneling activity of mole crickets. On damaged areas with high mole cricket infestation, 6–10 inches of the soil surface layer is honeycombed with numerous mole cricket galleries and the ground feels spongy underfoot. A severely damaged pasture has virtually no root system and the plants are easily pulled from the soil by grazing cattle. Research and surveys conducted throughout south-central Florida have shown a link between grazing intensity, declining soil pH, and severity of mole cricket-induced bahiagrass decline. Bahiagrass roots under low soil pH cannot absorb B, Mo, S, K, and P sufficiently, and damage caused by mole crickets can further stress the situation.
Field Study
Research was conducted at the Range Cattle Research and Education Center in Ona, FL, to evaluate the long-term effect of liming and N fertilization on dry matter yields, nutritive value, and ground cover of bahiagrass pastures. Treatments were a combination of four fertilizer treatments applied to bahiagrass pastures receiving lime and unlimed pastures. Control (no fertilizer application) and three fertilizer treatments applied in the spring were tested from 1998 through 2007. Treatments included in the study were: 1) 60 lb/acre of N from ammonium sulfate (N); 2) 60-25-60 lb/acre of N-P2O5-K2O from ammonium sulfate, triple super phosphate, and muriate of potash (NPK); 3) 60-25-60 lb/acre of N-P2O5-K2O plus 20 lb/acre of a micro-nutrients mix (Frit Industries, Inc.), which contained B, Cu, Mo, Fe, Mn, and Zn (NPKM); 4) no fertilizer control (Cont). Approximately one ton of dolomite was applied every three years to maintain soil pH around 5 on limed areas. Unlimed pastures exhibit soil pH of approximately 4.3. Bahiagrass dry matter yield, crude protein content, forage digestibility, and condition (color) of bahiagrass sod in the spring was determined annually during the 10-year study.
Dry Matter Yield
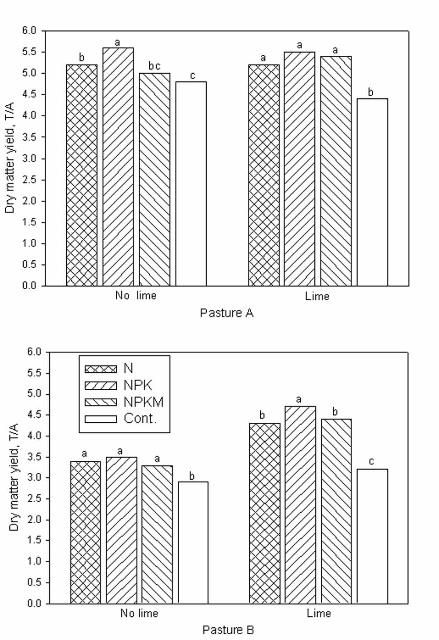
Pasture A probably had a slightly better soil buffering capacity because soil pH decline was minimal and dry matter yield was not significantly affected by liming to a pH of 5 (Figure 2). The no-lime plots on pasture A declined to a pH of about 4.5 during the study period. Soil pH of N-fertilized plots without lime declined to 4.3 in three years on pasture B, an elevated and well-drained site.
Liming plus N application (either from N, NPK or NPKM) improved bahiagrass yield by 30% over plots in pasture B that received N fertilizer with no lime (Figure 2). On average, the yield increase from N-fertilizer over the no fertilizer control for both pastures was about 25%. No significant yield response to P and K fertilization was observed under grazing on both pastures, although tissue P and K concentrations declined by 33% and 36%, respectively when these nutrients were omitted. Similarly, no yield response was observed when micronutrients were applied to bahiagrass pastures.
Nutritive Value
Lime application had little effect on bahiagrass crude protein concentration and digestibility. Conversely, in addition to yield increase, N application increased crude protein concentration by about 2% (12% versus 10%). The effect of N application on protein increase was more evident in the spring, immediately after the fertilizer application and generally decreased over time throughout the growing season. Forage digestibility for the no-fertilizer control was always the lowest (47%), although response of digestibility to N application was quite variable across the pastures and season.
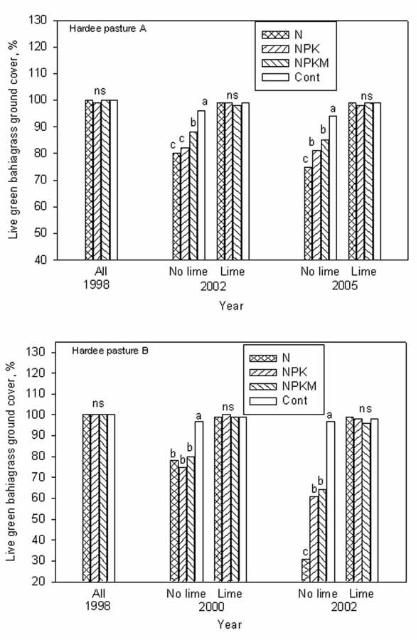
Effect of Lime and Fertilizer on Spring Sod Ground Cover
We observed that damage to bahiagrass sod was more pronounced when soil pH was below 4.5. At the beginning of grazing in the spring of 1998, all the newly established bahiagrass plots had excellent stands with nearly 100% green ground cover. Two years later (2000), the color of bahiagrass ground cover on pasture B began to decline in response to liming management and fertilizer treatments. Unlike the unlimed pastures, in 2002, five years after the experiment initiation, minimum spring color change or damage to bahiagrass sod (1%–4% ground cover consisting of yellow, brown, or weedy cover) was noticed for plots limed to pH 5 whether or not they received fertilizer, or for no-limed plots that were not fertilized (Figures 3 and 4). Sod damage was most severe (20%–69% of ground cover) when bahiagrass pasture was not limed but received yearly application of any N-containing fertilizer even at the rate of 50 lb N/acre. The combination of acidic soil conditions (pH less than 4.5) and repeated N fertilization reduced bahiagrass stolon/root biomass by 30%, weakened the root system, caused severe yellowing in early spring growth, and favored mole cricket damage and weed infestations. For pasture A, it took longer for the signs of damage to occur. In 2002, similar damage to sod was observed in pasture A as observed in pasture B in 2000 (Figure 4).
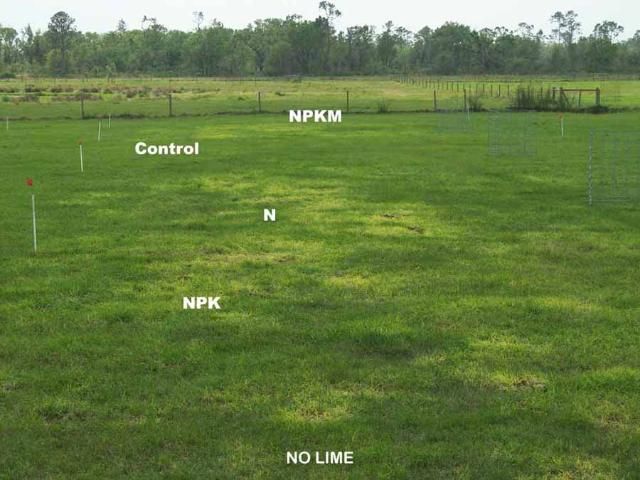
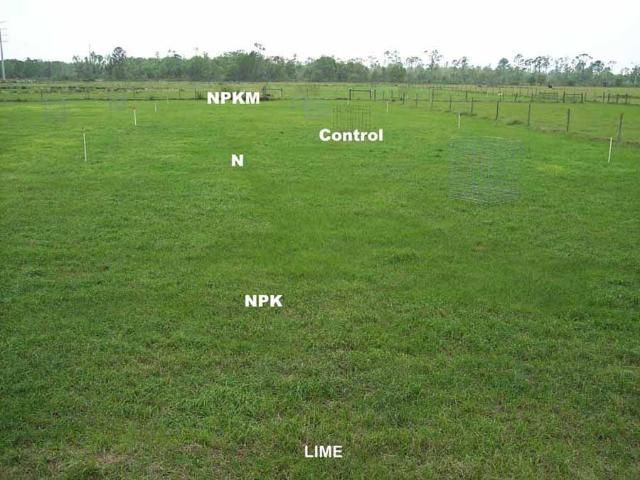
Effect of Biosolids on Bahiagrass Sod
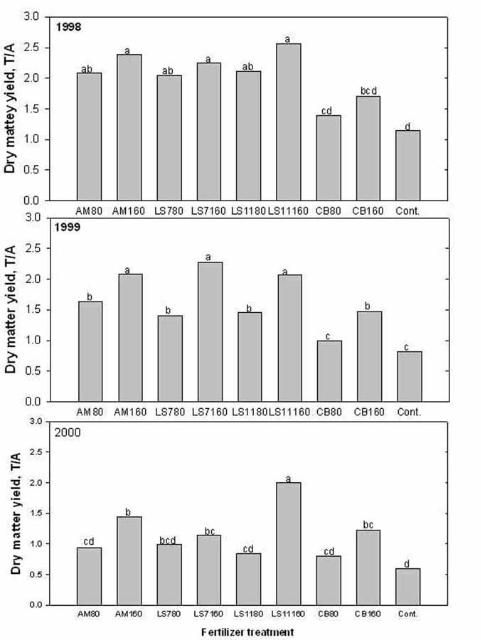
Some livestock producers apply lime-stabilized biosolids to pastures to reduce the cost of fertilizer and lime. Biosolids can be used as a source of plant nutrients (especially N and P), and organic matter to pastures. During the biosolids treatment process, lime can be added to control pathogens, insect vectors, and odor. Lime addition can also correct soil pH. The pH of lime-stabilized biosolids can range from 7 to 11. Nitrogen concentrations are usually between 3% and 5% (dry matter basis), and P concentration between 2% and 4%. Four consecutive years of repeated application of limed-biosolids at the Range Cattle Research and Education Center, Ona, FL, showed that when used at recommended agronomic rate (200 lb N/acre), bahiagrass forage production responded well to biosolids application. No sod damage was observed after repeated application of biosolids. Biosolids applied annually at 160 lb of N rate improved annual forage dry matter yield. Bahiagrass production was less than 1.0 ton/acre when no biosolids were applied compared to 2.5 ton/acre when biosolids were applied (Figure 5). There was no excessive buildup of plant nutrients or trace metals in the soil from repeated biosolids application, and soil pH increased from 5.0 to 5.3 in four years. However, several reports show that bahiagrass pastures in Polk, Pasco, and Hardee Counties received excessive amounts of lime-stabilized biosolids resulting in pH values greater than 7. Bahiagrass roots cannot function properly to absorb sufficient iron, manganese, and other micronutrients when the soil pH approaches 7, so those pastures lost substantial portions of grass sod to weeds in a manner similar to the symptoms of bahiagrass decline due to soil acidity.
Summary and Recommendations
Under grazing conditions in south-central Florida flatwoods:
- Apply 50 lb of N/acre to bahiagrass in early spring to boost yield and forage crude protein concentration.
- Bahiagrass forage yield often may not respond to P and K application under grazing. Tissue analysis in combination with soil test should be performed to identify whether or not P is needed.
- Repeated N fertilization with ammonium nitrate or ammonium sulfate can cause substantial decline in soil pH. Look for signs of early spring yellowing.
- When using N fertilizer, soil should be tested at least every 3 years. If pH is below 5.3, lime should be applied to correct soil pH. When applying lime/dolomite, be realistic and economical in choosing amount to apply. Apply a minimum of 1 ton of lime when soil test results recommend liming. Lower application rates may not be economical. Avoid overliming because high soil pH (> 7) negatively affects forage growth.
- When using lime-stabilized biosolids, apply material uniformly at the recommended agronomic rate. Soil test should be done at least every 3 years to avoid excessive soil pH.
- Because of their contrasting effects on soil pH, alternate the use of lime-stabilized biosolids with inorganic N fertilizer such as ammonium sulfate in order to maintain the optimum soil pH range of 5.0–6.0 and avoid accumulation of excess P.